Daub Marley
Active Member
Why do trichomes sink?
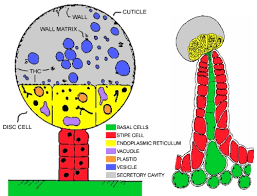
I mean oil floats on the surface of water and the trichome heads are made of non-polar chemicals just like the oil. The repelling forces in the oil ensures a great enough distance between the molecules that it becomes less dense than water and floats. So what’s going on here? Is the disc cell weighing it down, or is the inside of the trichome under pressure?
I mean oil floats on the surface of water and the trichome heads are made of non-polar chemicals just like the oil. The repelling forces in the oil ensures a great enough distance between the molecules that it becomes less dense than water and floats. So what’s going on here? Is the disc cell weighing it down, or is the inside of the trichome under pressure?