I had.. But he asked me what's the problem,? I don't have any.. I dont grow currently.. But I would like to hear more about how that buffering of soil works,in plain language.. Keep in mind eng is not my native language..
Great, plain language - gee thanks.....
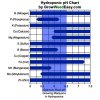
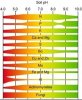
This is not going to be easy.....I'll guess you understand just what pH is = Potential hydrogen.
To make this easier on me, here is a simple educational site that may help explain basic soil pH...and what effects it's natural value....
http://passel.unl.edu/pages/informationmodule.php?idinformationmodule=1130447041&topicorder=6&maxto=10
Now to take that deeper as to the relationship of plant to soil to pH value..
Soils contain fungus and bacteria (living bio's). The spectrum of the type of bio's help form the basic pH value of the soil. Every plant has a "comfort" zone pH. By adding a plant to a soil. The plant exchanges hydrogen (+ charged) cations (ions of minerals) for a nutrient ion. This makes the hydrogen ions increase in the soil, thus lowering the pH. The effect of this is more pronounced in moist soil.
Now root surfaces also
take up - charged - nutrient ions. This action helps balance out that rise from the + charged exchange. This of course now raise's the pH.....You sort of get a balance but, you must remember that when wet., the - action is happening more and as it dries the + action is happening more -
to a point.
The plant helps "set" the soil it's in, pH value "at rest". The actions of the the plant and the soil briefly change the soils pH value when watered or when moisture in present vs not....
I should also mention that these fungi and bacteria have cations and anions (ions of minerals) on their surface. "electrically" holding or releasing these mineral nutrients they take in from decomposition of the soil. This too effects soil pH value.
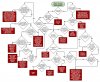
Now then. The plant effects what living bio's in the soil thrive and feed the plant. They mutually effect the soils pHto be favorable to the plant. This is part of what is called the SOIL FOOD WEB...
I hope that worked for you Disko! I'm not sure how to put it any simpler.....
Anyone got something to add?