My vegetative plants are infected with fungus gnats again.

Sigh. Now I'm adding 1/4 cup of 35% H2O2 in about a gallon of nutes, and I will continue that treatment for over a month, irrigate every 5 days for about 6 weeks. Supposedly kills their larve. I guess I'm gonna have to treat the household drains as well on a similar schedule since the gnats seem to be attracted to them, run the hottest tap water down the drain, followed by boiling-hot pan-boiled water seems less expensive than hydrogen peroxide. And they're attracted to pet's water dishes, so those also have to be throughly cleaned and refilled on fungus-gnat-treatment day. I put it in my calendar and I get notices that it's a day to treat for fungus gnats. Damn, I was just refilling a teapot at the kitchen sink, there's an adult fungus gnat flying around!
When I'm done with the treatments a month and a half from now, I'll put a tablespoon or two of earthworm castings as a potting mix topdressing to hopefully reinoculate the potting mix, maybe make a gallon of worm-castings tea and irrigate with it. Could earthworm castings have been the source of the gnat infection?
"the protective effects of the worm castings will help prevent diseases and keep fungus gnats under control."
Hmm, I actually forgot to add worm castings in this last batch of potting mix. When I did use it a few seasons ago, I didn't have a problem with gnats. I guess I need another list of *ingredients to include in potting mix* so I don't forget on potting-mix mixing day. If that doesn't fix fungus gnats next season, then I'll have to include some diatomaceous earth in the mix. I'd rather not have to continually use H2O2, it's relatively expensive.
My vegetative plants have some curious yellowing, beginnings of yellowish splotches. One of the oldest leaves is mostly yellow, with a dark stem.
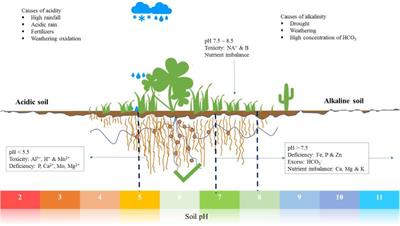
I posted a pic in Marijuana Plant Problems and asked for suggestions. From diagnosis charts I've collected over the years, that appears as either iron or sulfur deficiencies. My nute formulas are based upon hydroponic tomato fertilizer 4-18-38, that I have additionally supplemented with calcium nitrate, epsom salts, MKP and sometimes MAP to alter NPK ratios; and a pH adjuster, in the past I've used hydrated lime, but I've moved to KOH.
According to the
Aquaponics Association, potassium silicate and calcium carbonate are preferable chemicals for pH Up. Interesting. I know I've seen members here mention potassium silicate, though I don't believe the mentions were in the context of pH adjustment, rather the addition of silicates.
My formulas are weight-based notations, as I've cut down the EC (fertilizer strength), there's as little as 1/5 the weight of 4-18-38 required for a full dose of micronutes, so I supplement the missing portion with a corresponding portion of soluble trace element mix, S.T.E.M. Upon rereading the S.T.E.M. label, I can increase its strength 50% and still be a label recommended amount, so that will be my first stab at correction, along with 2 other adjustments.
Here's an interesting little factoid I did note in the course of troubleshooting this plant's distress. Peter's S.T.E.M. powder uses metal sulfates, while their liquid S.T.E.M. uses metal EDTAs, i.e., chelated metals. I'm using the powdered sulfate type, so unfortunately iron and manganese are less available at higher pH than if they were chelated. With the items I currently have on hand, the gallon of B-1 is my only existing source of iron EDTA which is supposedly available at up to 7.0 pH.
My last irrigation the nute's EC was diluted to 1300 (µS/cm), but my last runoff had a final measurement of 2100. Therefore, for next watering I'll reduce nutes EC to 1200, hopefully so that runoff should be at or below 2000.
No matter how carefully I measure the powders for a gallon of nutes, the resulting EC seems to vary ± 100 µS/cm. Therefor, I aim to initially mix it (per gallon) slightly stronger than desired, and dilute to desired EC, always giving me somewhat more than a gallon.
I was running the pH of vegetative formulas somewhere around 6.3 to 6.5, my pH meters aren't that accurate and different ones give me somewhat different readings (I use two at the same time which gives me a range, and I recalibrate between every measurement). Given that the powdered S.T.E.M. only uses metal sulfates, I need to lower that to 6.0 to 6.3 pH for better availability to the plant. This last fertilization several days ago I lowered the nute's pH, maybe as low as 6.0. One meter says 6.0, the other 6.3, so hopefully it's somewhere within that range.
I have also replaced the calnit with a 50:50 calnit/ammonium sulfate mix, which shouldn't be as alkaline forming, and will supply more
sulfur. So, lots of options, first I'll try what I can without additional purchases.
➤ Too little S.T.E.M powder (now increased to highest label-recommended rate, but this is not chelated iron, only metal sulfates)
➤ Lower the pH of vegetative fertilizer solutions to nearer 6.0 (so existing iron sulfate is more available). Done henceforth within limits of imperfect pH-measurement tools.
➤ Drainage pH was higher than 7.0 pH, which is too alkaline, nor do I add lime to my potting mix, so I presume that is all from alkaline forming nitrogen. I probably want drainage pH somewhere around 6.0 to 6.3. I've replaced some calcium nitrate with ammonium sulfate for a less-alkaline and more-acid nitrification. My first value is roughly 50:50 calnit:ammonium-sulfate, but I need to go through at least one irrigation cycle for the acidification or nitrification to occur and hopefully be measured as a lower drainage pH. That will also up the sulfur content, in case it is sulfur and not iron deficiency. I presume there will be further ratio changes, I'm hoping that whatever ratio is found will work similarly for all my vegetative formulas.
A further tactic I could implement:
➤ Switch from the newer 3-1-1 (NPK) formula to a 3-1-2, the idea being the higher potassium should help iron absorption, from Mulder's chart, synergy between potassium and Fe.
I've recently learned of
Iron DTPA, which is claimed as actively-available at higher pH values than EDTA-chelated iron. That may go on a purchase-wish list along with potassium silicate.
In tracking down the source of this vegetative nute problem, I found the Peters S.T.E.M. powder is perhaps the cause of the yellowing, nutes with lower pH and less alkaline forming N will hopefully fix it. I'm considering purchasing EDTA, to chelate the powdered S.T.E.M. sulfates myself during mixing. The longer term problem I'm perceiving is that during bloom, optimal pH is higher than for vegetative, so I will need to find a way to insure micronutes are all available to the plant during bloom cycles.
This has been an interesting problem. 4-18-38 uses EDTA-chelated micronutes. It is supposed to be used at a rate of 2.4 g/gal. In my NPK-modified mixtures, as I've reduced its portion for reasons of EC (2.4 to 0.5 g/gal) and supplemented the associated micronute losses with Peters powdered S.T.E.M, I downgraded micronutes to non-chelated sulfate forms, of which iron and manganese both begin to lose absorbability above 6.0 pH.
It's always nice to understand why a growth problem occurs!
As I'm continuing to think about this, purchasing some EDTA and chelating the micronutes myself seems a reasonable course of action. If I can get drainage pH to the perfect zone, 6.0 to 6.3, chelation probably won't be needed, but I've read that bloom has a higher optimal pH range, 6.5 to 6.8 or thereabouts. So unless I figure out how to get micronutes available at that higher pH level, then my blooms will be less than optimized at best. Thus the desire to chelate the metal sulfates! An alternative is to obtain Peters Liquid S.T.E.M., but they don't sell small quantities, their 2.6x gal jugs are for huge commercial outfits growing lots of plants, and I can't justify purchasing that much at once.






 Sigh. Now I'm adding 1/4 cup of 35% H2O2 in about a gallon of nutes, and I will continue that treatment for over a month, irrigate every 5 days for about 6 weeks. Supposedly kills their larve. I guess I'm gonna have to treat the household drains as well on a similar schedule since the gnats seem to be attracted to them, run the hottest tap water down the drain, followed by boiling-hot pan-boiled water seems less expensive than hydrogen peroxide. And they're attracted to pet's water dishes, so those also have to be throughly cleaned and refilled on fungus-gnat-treatment day. I put it in my calendar and I get notices that it's a day to treat for fungus gnats. Damn, I was just refilling a teapot at the kitchen sink, there's an adult fungus gnat flying around!
Sigh. Now I'm adding 1/4 cup of 35% H2O2 in about a gallon of nutes, and I will continue that treatment for over a month, irrigate every 5 days for about 6 weeks. Supposedly kills their larve. I guess I'm gonna have to treat the household drains as well on a similar schedule since the gnats seem to be attracted to them, run the hottest tap water down the drain, followed by boiling-hot pan-boiled water seems less expensive than hydrogen peroxide. And they're attracted to pet's water dishes, so those also have to be throughly cleaned and refilled on fungus-gnat-treatment day. I put it in my calendar and I get notices that it's a day to treat for fungus gnats. Damn, I was just refilling a teapot at the kitchen sink, there's an adult fungus gnat flying around!