You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Phosphor conversion based LED limit......
- Thread starter Stephenj37826
- Start date
JorgeGonzales
Well-Known Member
Because more photons, but not more energy? I swear I keep saying the same thing, if I'm wrong someone correct me lol.
Well no, that makes total sense to me, but I guess I'm wondering if the energy lost in conversion has any relationship with the distance shifted.
JorgeGonzales
Well-Known Member
Is it just a matter of equal quantum yields(?) in red phosphors at different wavelengths, like 600nm vs 630nm? Have things improved in recent years?
Atulip
Well-Known Member
Should just depend on the efficiency of the material, I can see more photons out than in in theory. Some of this stuff gives me a headache trying to understand.
Speaking of different wavelengths photon energy, how does that relate to photosynthesis. 630 vs 660, etc? And since we compare lights with photon count rather than energy...? I'm sure it's been discussed here somewhere, someone point me in the right direction lol.
Speaking of different wavelengths photon energy, how does that relate to photosynthesis. 630 vs 660, etc? And since we compare lights with photon count rather than energy...? I'm sure it's been discussed here somewhere, someone point me in the right direction lol.
robincnn
Well-Known Member
https://en.wikipedia.org/wiki/Jablonski_diagram

So the key here is 1 Blue photon converts to 1 Red or yellow Photon. The energy difference is lost as Stokes shift loss
It cannot take 2 blue photons and then give 3 red photons. It does not work like that.

I think the way it works is that a high energy (hv) blue photon hits and electron and pushes it to higher energy state. Now some of this energy (E) is lost in molecular vibrations what i do not completely understand. So now electron jumps back to default state and emits a lower energy (hv-E) red/yellow photon.I thought we were absorbing photons, then using the energy to create new photons. The only limit should be the amount of energy.
So the key here is 1 Blue photon converts to 1 Red or yellow Photon. The energy difference is lost as Stokes shift loss
It cannot take 2 blue photons and then give 3 red photons. It does not work like that.
Last edited:
pookat
Well-Known Member
a bit late......slow internet
like switches or buttons, each light wave nm fits a specific receptor, like russian stacking dolls fit into each other red,yellow,blue & violet. so Red + yellow = orange etc. for photosynthesis it has specific receptors for the primary light and the various blended light activated by the light waves "fatness" ie. 400nm+600nm=greeny color or fat blue/skinny yellow or fat yellow/thin red is 600nm+800nm = orangey.
how does that relate to photosynthesis. 630 vs 660, etc?
like switches or buttons, each light wave nm fits a specific receptor, like russian stacking dolls fit into each other red,yellow,blue & violet. so Red + yellow = orange etc. for photosynthesis it has specific receptors for the primary light and the various blended light activated by the light waves "fatness" ie. 400nm+600nm=greeny color or fat blue/skinny yellow or fat yellow/thin red is 600nm+800nm = orangey.
robincnn
Well-Known Member
Yes and Goud is not helpingSome of this stuff gives me a headache trying to understand.
ok i will read Alesh's post again.what is the QER of a white Cob?
https://www.rollitup.org/t/math-behind.868988/#post-11542395
Well no, that makes total sense to me, but I guess I'm wondering if the energy lost in conversion has any relationship with the distance shifted.
Source:
https://www.osapublishing.org/view_article.cfm?gotourl=https://www.osapublishing.org/DirectPDFAccess/BFBD2452-0052-8C51-A8E23DB4DA2DA9B7_251966/josk-17-1-22.pdf?da=1&id=251966&seq=0&mobile=no&org=
I do not understand the whole math there.
Lets look at equation 11
So from this equation it is easy to understand that stokes loss in phosporus is
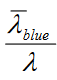
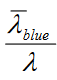 Means you will have more stokes loss for 90 CRI as it has more 660 red. 450/ 630 vs 450/660
Means you will have more stokes loss for 90 CRI as it has more 660 red. 450/ 630 vs 450/660Although you have more stokes loss, the number of photons is still the same....
assuming both red and yellow Phosphorus have same
Attachments
Last edited:
Stephenj37826
Well-Known Member
https://en.m.wikipedia.org/wiki/Fluorescence
This actually puts what I've been theorizing into scientific fact lol. Geez I should use Google more often lol.
This actually puts what I've been theorizing into scientific fact lol. Geez I should use Google more often lol.
So photoluminescence, phosphorescence, and fluorescence, so we're not actually absorbing the photon energy as I was thinking, we're squishing the wave and robbing energy from the blue or uv photon?
Google never gives me a straight answer, what happened to Jeeves?
a billiard ball transfer of energy ?
Greengenes707
Well-Known Member
So the question lays around now at what is the current blue die efficiency at the ~100ma they are ran. And then we can see what the current level of phosphor loss on say a 3590 is.
1400ma=~100ma per die on a 3590.
Where is phosphor tech now, and where could it go. We know where blue dies are.
I asked for a big pure blue 3590 a year and a half ago...could have come in handy for a comparison like this.
1400ma=~100ma per die on a 3590.
Where is phosphor tech now, and where could it go. We know where blue dies are.
I asked for a big pure blue 3590 a year and a half ago...could have come in handy for a comparison like this.
guod
Well-Known Member
So the question lays around now at what is the current blue die efficiency at the ~100ma they are ran. And then we can see what the current level of phosphor loss on say a 3590 is.
according to the Cree PCT the Xte royalblau (25°C -/- 100mA), the highest bin reaches 72%
phosphor loss is about 15% for high CCT and low CRI., and goes down to 35% for lower CCT and higher CRI.
 for royal blue and photo red Lumen is here milli Watt!
for royal blue and photo red Lumen is here milli Watt!Stephenj37826
Well-Known Member
according to the Cree PCT the Xte royalblau (25°C -/- 100mA), the highest bin reaches 72%
phosphor loss is about 15% for high CCT and low CRI., and goes down to 35% for lower CCT and higher CRI.
View attachment 3702768 for royal blue and photo red Lumen is here milli Watt!
What is LER of royal blue??????? Something doesnt add up with that many lumens a watt in royal blue....... it should be impossible to get that number with a 100% efficient 555 nm green.......
Stephenj37826
Well-Known Member
Is that mw/watt?
It says lm/W lol. Maybe so. Actually I take that back I think so lol.
Johnnycannaseed1
Well-Known Member
That's kinda what I was getting at. As I still ponder can I hit the phosphor with x photons in any nm and get more photons out as in numerically? Just curiosity lol. Is it possible or is that the limitation of the phosphor itself. As much as I do understand some of this it still leaves me wanting to understand the exact reactions that take place inside the phosphor. It may be a question similar to "how many licks does it take to get to the center of a tootsie pop?" Crunch the world may never know lol.
Because more photons, but not more energy? I swear I keep saying the same thing, if I'm wrong someone please correct me lol.
After reading your posts thinking you would both be interested in the principles of photon phosphor upconversion because it seems to answers what you appear to be alluding to, albeit there are current limitations, choice of wavelength applied being one of them
http://www.nature.com/ncomms/2015/151116/ncomms9920/full/ncomms9920.html
sixstring2112
Well-Known Member
That's kinda what I was getting at. As I still ponder can I hit the phosphor with x photons in any nm and get more photons out as in numerically? Just curiosity lol. Is it possible or is that the limitation of the phosphor itself. As much as I do understand some of this it still leaves me wanting to understand the exact reactions that take place inside the phosphor. It may be a question similar to "how many licks does it take to get to the center of a tootsie pop?" Crunch the world may never know lol.
Thats an easy one.its 3 licks,ah 1, ah 2, ah 3. Three lol.alot of the younger crowd here wont get that
JorgeGonzales
Well-Known Member
Thats an easy one.its 3 licks,ah 1, ah 2, ah 3. Three lol.alot of the younger crowd here wont get that
That's worth sharing
Similar threads
- Replies
- 71
- Views
- 9K
- Replies
- 13
- Views
- 1K
