MrPuffTuff
Active Member
So, while trying to understand the different forms of nitrogen and how they relate, and what can be used where, I've come across a few links that have helped quite a bit.
I am curious as to other's understanding of this and how they have applied this knowledge in choosing nutrients.
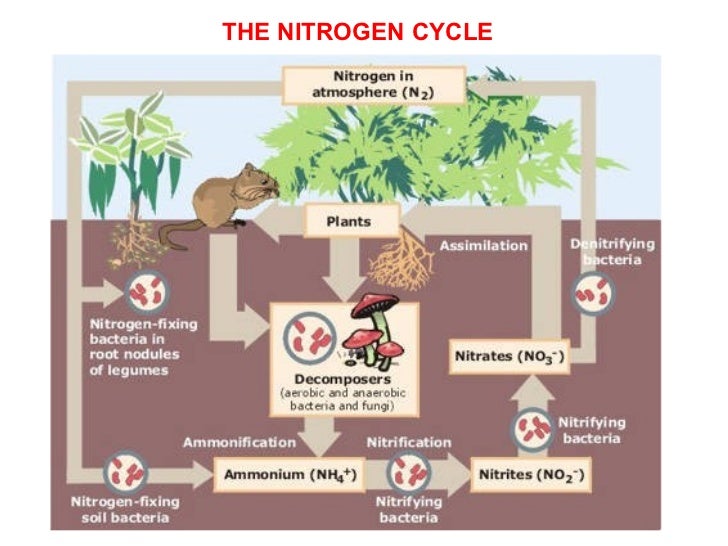
I believe many out there just see the NPK ratios, and go straight from there assuming the N number tells them how much nitrogen they are providing their plants. This seems to be wrong, however - as ammoniacal nitrogen (ammonium, often derived from ammonium sulfate) is not readily available to plants and requires to be broken down by microbes into nitrates before being assimilated by the plant - meaning ammoniacal N is fine for growing in soil. However, hydroponic nutrients usually have 90% nitrate-form N, due to there being little to no microbe activity in hydroponic solutions as opposed to being in soil. I grow in both, so learning more about this has helped immensely.
This topic is also important for those sticking to an all-organic regimen. There's another common source of nitrogen in products out there, plant protein hydrolysate, which seems to act as more of a bio-stimulant as opposed to a nitrogen source (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5744479/). Products derived from this show an NPK with a significant N percentage, but the nitrogen present is not available for uptake by the plant as far as I understand...
Anyone else have any input or disagreements? I'm trying to wrap my head around this topic and thought I'd share what I've found to see what others have to say.
more links:
https://www.growjourney.com/5-facts-synthetic-nitrogen-fertilizer/#.W5_wquhKhGM
(lightning actually feeds plants!)
I am curious as to other's understanding of this and how they have applied this knowledge in choosing nutrients.
I believe many out there just see the NPK ratios, and go straight from there assuming the N number tells them how much nitrogen they are providing their plants. This seems to be wrong, however - as ammoniacal nitrogen (ammonium, often derived from ammonium sulfate) is not readily available to plants and requires to be broken down by microbes into nitrates before being assimilated by the plant - meaning ammoniacal N is fine for growing in soil. However, hydroponic nutrients usually have 90% nitrate-form N, due to there being little to no microbe activity in hydroponic solutions as opposed to being in soil. I grow in both, so learning more about this has helped immensely.
This topic is also important for those sticking to an all-organic regimen. There's another common source of nitrogen in products out there, plant protein hydrolysate, which seems to act as more of a bio-stimulant as opposed to a nitrogen source (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5744479/). Products derived from this show an NPK with a significant N percentage, but the nitrogen present is not available for uptake by the plant as far as I understand...
Anyone else have any input or disagreements? I'm trying to wrap my head around this topic and thought I'd share what I've found to see what others have to say.
more links:
https://www.growjourney.com/5-facts-synthetic-nitrogen-fertilizer/#.W5_wquhKhGM
(lightning actually feeds plants!)

