related backcrossing info:
Backcrossing wild tomatoes
http://onlinelibrary.wiley.com/doi/10.1111/tpj.13194/full
Mouse strain, line classification etc.
http://www.informatics.jax.org/silver/chapters/3-2.shtml
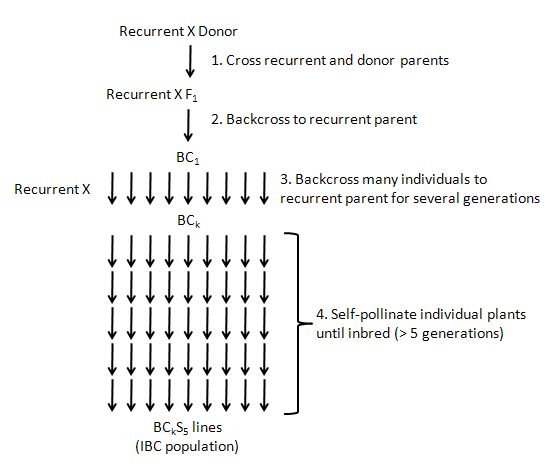
Fantastic overview of Backcrossing
http://passel.unl.edu/pages/printinformationmodule.php?idinformationmodule=959723462
Strains: A strain is a partially reproductive isolated group of organisms. A strain generally differs from some other strain in the mean response for some trait. A very amorphous term. A new strain of Drosophila, for example, might be established by collected a few flies from the wild and allowing then to interbreed. Might also be called a line, a stock or a variety.
Isofemale line: Common in Drosophila. A single gravid female is collected from the wild. Her progeny are allowed to interbreed. This line will be partially inbred since it will be founded with a the genes from a single female and from as few a one male.
Inbred strain: An Inbred strain is one that is homozygous at every locus and the alleles at each locus are identical by descent.
Inbred strains take 20 generations of brother-sister matings to achieve. Starting with a single ancestral breeding pair, a brother and sister are chosen each generation. Alternatively, inbred lines can be established by mating offspring back to a parent in a regular pattern. Occasionally, other systems of inbreeding might be used but a line cannot be considered inbred until it reaches an inbreeding coefficient equal to 20 generations of brother sister matings (99% or better). Inbred lines generations are often designated as F generations (F3, F10, F20 etc.)
Substrain: Two inbred lines from a common origin may be considered substrains. This implies that the substrains differ at several loci. If two inbred lines are separated before 40 generations of inbreeding, there will still be enough heterozygosity (less than 1% of the genes would vary) at separation that two genetically different substrains could result.
Alternatively, genetic variation between the branches may have occur by mutation and genetic drift. If the variation is at a single locus this would be a coisogenic line (see below). If it is at several loci, it is a substrain. Finally, if variation between isolated branches of an inbred line can be demonstrated (per haps my microsatellite analyses) they the branches can be considered substrains.
Recombinant inbred (RI) strains are formed by crossing two inbred strains to make an F1 generation. Brother and sister pairs from this F1 generation are used to establish many different inbred lines. These RI lines require 20 or more generations of brother-sister matings.
The RI lines are started from a limited genetic pool. The original inbred lines differed at a number of loci. The RI lines differ at an intermediate number of loci when compared to the progenitor strains.
RI strains are designated by an abbreviation of both parental strain names separated by a capital X. For example, CXB, are recombinant inbred strains derived from a cross of BALB/c x C57BL.
Congenic Strains: The idea is to obtain strains that differ by only a few specified genes (ideally by a single gene). Usually, two inbred lines are crossed to make an F1 generation (here, however, it will be called an N(1) - see below). The F1 progeny are backcrossed to one of the parental inbred lines. These N(2) progeny will now have 75% of their material derived from a single parental line. N(2) progeny are again backcrossed to the same parental line to make an N(3) generation, etc. It takes 10 generations of backcrossing for a line to be considered as congenic.
After 10 generations, the congenic line will differ from the parental line by the gene of interest and a short linked chromosomal segment around that gene.
Speed Congenics: It takes a minimum of 10 generations to get a congenic line. In mice, this is approximately 2.5 years. Speed congenics are an attempt to cut that time.
Speed Congenics are merely a variation on regular congenics. At generation N(2) individual mice (generally males) are genotyped for 60 to 100 microsatellite markers and the mice with the that have the greatest share of the inbred background chromosomes you are interested in are selected as parents. (Males are used since a single male can be backcrossed to many different Parental strain females). This processes may eventually be further accelerated by microarray screens.
The savings can be considerable. Speed congenic technology might give you a congenic strain in 12 to 18 months versus 2 to 3 years.
Coisogenic Strains: Two strains that are genetically identical (i.e. isogenic), except for a single locus. This occurs most often by a spontaneous mutations by many generations of backcrossing. Coisogenic strains are also becoming available due to target mutagenesis (knock-outs) in Embryonic Stem cells (ES). For example, a target mutation is induced in an inbred strain (e.g. 129SV) and the mutant mice are backcrossed repeatedly to the same inbred substrain from which the ES cells were derived would be a coisogenic line. but the possibility of mutations elsewhere should be considered. Similarly, chemically or radiation induced mutants in an inbred background can be considered coisogenic (but only if other mutations are not present).
Recombinant Congenic (RC) A combination of congenic and inbred lines. Two inbred lines are crossed to form an F1. The F1 progeny are backcrossed to one of the parent lines for Strains are formed by crossing two inbred strains, followed by a few (usually two) backcrosses to one of the parental strains (the "background" strain), with subsequent inbreeding without selection for specific. Such a strain is developed by crossing male C57BL/6J mice with BALB/c females, followed by repeated backcrossing of female offspring to male C57BL/6J.
As with congenic strains, a minimum of 10 backcross generations is required, counting the F1 generation as generation 1. For full inbred status, however, you need 20 inbred generation equivalents
One generation of backcrossing is equivalent to two generations of brother-sister matings. Thus, a strain with one backcross [N(2)] is equivalent to a strain with 4 generations of inbreeding F4. For full inbred status, it would require an addition 16 generations of brother sister matings.
Segregating inbred strains: are developed by inbreeding with but one or more loci must remain heterozygous. This generally requires progeny screening each generation and selection of heterozygous parents.
As with any inbred strains it takes 20 generations of brother sister mating. These lines are designated as FH lines to distinguish them from homozygous inbred lines.
Consomic strains are produced by repeated backcrossing of a whole chromosome such as the X or Y chromosome onto an inbred strain. As with congenic strains, a minimum of 10 backcross generations is required.
Two strains A and B are crossed. In the cross A x B the F1 male progeny would have their X chromosome from the A strain and the Y chromosome from the B strain. The autosomes are from both strains. With repeated backcrossing to the A you will obtain a line of animals that have all A-strain autosomes, A-strain X-chromosome and B strain Y-chromosome. Similar methods can be used to get X chromosomes into different backgrounds
The strains are designated as host stain - chromosome (X or Y) and the donor stain. In the example above, this strain would be A-YB