Hot Diggity Sog
Well-Known Member
This is probably going to turn into a lengthy discussion but I would like to 1st discuss an obvious problem I am seeing. Maybe I'm missing something...I don't know.
Bit of background first - I've always been a bottled nutes guy but I've spent the last two months reading as much as I could to learn about making my own nutes.
I mixed up my first test batch and my numbers are nowhere close to what HydroBuddy predicted they would be - but more on that later. I would like to start with the following problem I see in HydroBuddy:
Lets take the following recipe: (the ingredients are actually irrelevant)
Note: The water quality parameters are all set to 0.

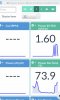
All of the PPM amounts in HydroBuddy are on the 500 scale. If you add up all of the PPM's from the lower left table, you get ~ 667 PPM.
The predicted EC value is shown in the lower right at 1.485. But if you do the math and divide 667 / 500 you get 1.334 EC.
1.33 is way off from the predicted 1.485. I don't understand. 1.485 EC would be ~ 743 PPM. That's a 76 PPM difference.
Can anyone explain this to me?
Bit of background first - I've always been a bottled nutes guy but I've spent the last two months reading as much as I could to learn about making my own nutes.
I mixed up my first test batch and my numbers are nowhere close to what HydroBuddy predicted they would be - but more on that later. I would like to start with the following problem I see in HydroBuddy:
Lets take the following recipe: (the ingredients are actually irrelevant)
Note: The water quality parameters are all set to 0.

All of the PPM amounts in HydroBuddy are on the 500 scale. If you add up all of the PPM's from the lower left table, you get ~ 667 PPM.
The predicted EC value is shown in the lower right at 1.485. But if you do the math and divide 667 / 500 you get 1.334 EC.
1.33 is way off from the predicted 1.485. I don't understand. 1.485 EC would be ~ 743 PPM. That's a 76 PPM difference.
Can anyone explain this to me?
Last edited: