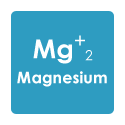ClassicT
Active Member
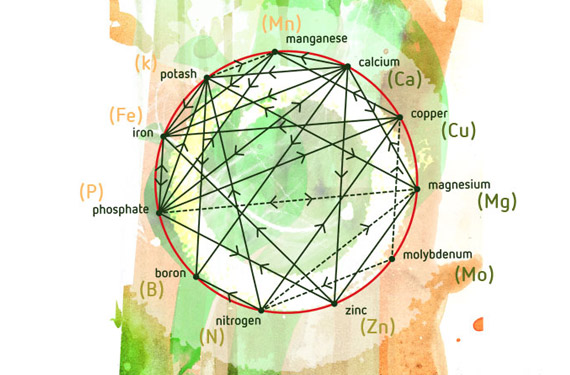
My plants have been teaching me a lot lately! Mostly, that nutrients work in specific tandem with one another and therefore straying too far outside of a particular manufacturer’s nutrient line and feeding schedule has a big potential to cause nutrient lockouts and deficiencies.
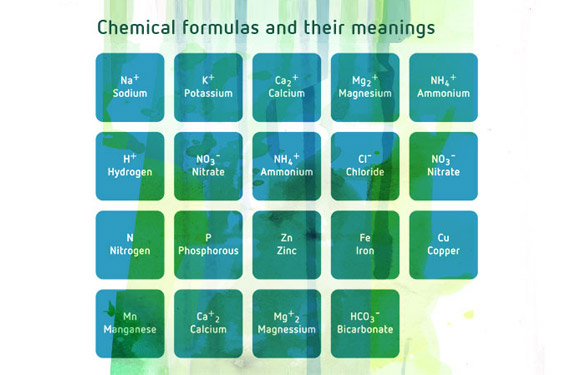
In my case, my ladies are in week 5 of flower, and have a major excess of phosphorous and a major deficiency of nitrogen. I found this from doing one of those RapiTest chem tests with the little vials and powders. I definitely overdid it on the bloom nutes and boosters a few weeks ago.
Of course, each plant is performing slightly differently. 2 of the 9 have very light lime green leaves on the top of the canopy (a result of bleaching caused by excess calcium I believe) and the other seven look pretty healthy. They ALL have purplish/red petioles, and they all have very very very very minor tip burn/yellowing. All have good and consistent bud growth, even the lime colored ones, which is a relief.
But even the healthy looking ones showed barely any detectable nitrogen in my soil test.
I watered with plain water last time, and got a nice pH 6.5 for my runoff.
Now I’m trying to determine what to include in my next water. N deficiency is confirmed, P excess is confirmed, and I suspect the purple petioles throughout indicate Mg deficiency. (K tested as “medium” amount.)
So I was planning to add a regular (regular meaning the low end of what the bottle label says to add at this stage) amount of a (0.5 - 0 - 0) to the ones that are the palest lime green, half that amount of the (0.5 - 0 - 0) to the ones that look slightly lime green, and the low end of the recommended amount of a (2 - 1 - 1) to the healthy looking ones.
Plus a teaspoon/gal of Epsom for all of them for the suspected Mg deficiency.
Does anyone have experience with correcting deficiencies and lockouts with bottled nutrients? Is is best to give the plant what it is lacking or is it better to get back on track with plain water for two weeks and then get back on track with a full feed of everything in your particular nute line?
It makes sense to me to add what they are lacking, and avoid adding what they have in excess, but my “thinking” was what got me in this mess to begin with.
I’d love some feedback on your tales of recovering from deficiencies and lockouts, specifically in soil with bottled nutes. Thanks all!
In my case, my ladies are in week 5 of flower, and have a major excess of phosphorous and a major deficiency of nitrogen. I found this from doing one of those RapiTest chem tests with the little vials and powders. I definitely overdid it on the bloom nutes and boosters a few weeks ago.
Of course, each plant is performing slightly differently. 2 of the 9 have very light lime green leaves on the top of the canopy (a result of bleaching caused by excess calcium I believe) and the other seven look pretty healthy. They ALL have purplish/red petioles, and they all have very very very very minor tip burn/yellowing. All have good and consistent bud growth, even the lime colored ones, which is a relief.
But even the healthy looking ones showed barely any detectable nitrogen in my soil test.
I watered with plain water last time, and got a nice pH 6.5 for my runoff.
Now I’m trying to determine what to include in my next water. N deficiency is confirmed, P excess is confirmed, and I suspect the purple petioles throughout indicate Mg deficiency. (K tested as “medium” amount.)
So I was planning to add a regular (regular meaning the low end of what the bottle label says to add at this stage) amount of a (0.5 - 0 - 0) to the ones that are the palest lime green, half that amount of the (0.5 - 0 - 0) to the ones that look slightly lime green, and the low end of the recommended amount of a (2 - 1 - 1) to the healthy looking ones.
Plus a teaspoon/gal of Epsom for all of them for the suspected Mg deficiency.
Does anyone have experience with correcting deficiencies and lockouts with bottled nutrients? Is is best to give the plant what it is lacking or is it better to get back on track with plain water for two weeks and then get back on track with a full feed of everything in your particular nute line?
It makes sense to me to add what they are lacking, and avoid adding what they have in excess, but my “thinking” was what got me in this mess to begin with.
I’d love some feedback on your tales of recovering from deficiencies and lockouts, specifically in soil with bottled nutes. Thanks all!
Last edited: