A guide to different pH down options in hydroponics – Science in Hydroponics
 scienceinhydroponics.com
scienceinhydroponics.com
A guide to different pH down options in hydroponics
The first group of pH down chemicals are strong acids. These are technically acids with very low pKa values, meaning they react instantly with water to generate at least one mole of hydronium for each mole of added acid. They offer the strongest ability to drop pH per unit of volume, which makes them more cost effective. However the fact that they often need to be diluted to make the pH addition process practical – because of how much the concentrated forms can change pH – can make their use more difficult than other forms of pH down. These are the most common options:
Phosphoric acid (from 20 to 85% pure): This acid doubles as a plant nutrient, meaning plants will be affected by the phosphorus added. It is commonly used in food – so food grade phosphoric acid can be bought cheaply – it also has additional deprotonations with strong buffering at a pH value of 7.2 with buffering capacity against bases getting stronger as the pH goes down all the way to 6.2. This is the most commonly used acid by hydroponic growers.
Sulfuric acid (from 20 to 98% pure): This acid is commonly used in car batteries and offers the largest pH dropping ability per unit of volume among all the strong acids. It is however important to use food grade sulfuric acid in hydroponics as normal battery acid can include some heavy metal impurities – from the fabrication process of sulfuric acid – that might negatively affect a hydroponic crop. Food grade sulfuric acid is safe to use in hydroponics. A big advantage is that plants are quite insensitive to sulfate ions – the nutrient provided by sulfuric acid – so adding sulfuric acid does not really affect the nutrient profile being fed to the plants.
Nitric acid (from 30-72% pure): This acid also provides nitrate ions to plants, so it also contributes to a solution’s nutrient profile. It is however more expensive than both phosphoric and sulfuric acids and more heavily regulated due to its potential use in the fabrication of explosives. The acid itself is also a strong oxidant, so storage and spillage problems are significantly worse than with phosphoric and sulfuric acid. Although this acid can be used in hydroponics, it is generally not used by most growers due to the above issues.
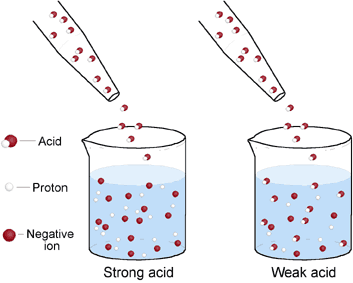
The second group of pH down chemicals are weak acids. These are acids that do not generate at least one mole of hydronium ions per mole of acid when put in solution, but do provide a pH down effect as some hydronium ions are generated. This means that larger additions will be needed to cause the same effect but at the same time their handling is usually much safer than for strong acids. Here are some options that could be used as a pH down.
Common food grade organic acids (citric acid, acetic acid, etc): Organic acids are a very low cost way to lower the pH of a hydroponic solution as many of these are available off the shelf in super markets in food grade qualities. The main issue with organic acids – which anyone who has used them has probably experimented – is that the effect of the acids does not seem to hold (pH goes up quickly after the acid is added and the solution comes into contact with plants). This is actually caused by the fact that plants and microbes can actually use the conjugated bases of these ions nutritionally, causing an increase in pH when they do so. The initial addition of say, citric acid, will drop the pH – generating citrate ions in the process – these will then be absorbed by microbes and plants, increasing the pH again rapidly. The use of these acids is therefore not recommended in hydroponics.
Monopotassium phosphate (MKP): This salt contains the first conjugate base of phosphoric acid and is therefore way less acidic than it’s full on acid partner. Since it’s a solid its addition is way easier to control compared to the acid and it can also be handled safely with minimal precautions. It provides both potassium and phosphorous to a solution – both important nutrients – and therefore needs to be used carefully when used as a pH down agent (as it significantly affects the nutrient profile of the solution). Since it adds both a cation that helps counter pH increases by plants and phosphate species it provides a double buffering effect against future pH increases. It is a very common ingredients of commercial pH down solutions for this reason.
Monoammonium phosphate (MAP): Similar to the above, except for the fact that this salt adds nitrogen as ammonium, which is a nitrogen form plants are very sensitive to. Plants will uptake ammonium preferentially over any other cation, so MAP provides a very strong buffering effect against nitrate absorption, with potential problems if too much is used (although this depends on the plant species being grown). When MAP is used as a pH down its addition therefore needs to be carefully controlled in order to avoid excess usage. Due to the presence of this powerful ammonium buffer, MAP is generally very effective at preventing future increases in pH, although this might be at the expense of yields or quality depending on the crop.
Potassium bisulfate: This salt contains the first conjugate base of sulfuric acid and is therefore a powerful tool to decrease the pH of a solution. The resulting sulfate ions provide no chemical buffering effect, so the only buffering effect in terms of plant absorption comes from the addition of potassium ions, which can help mitigate nitrate absorption. This salt is also considerably expensive compared with the two above – which are commonly used fertilizers – and is therefore seldom used in hydroponics.
Which is the best pH down solution? It depends on the characteristics of the growing system. Generally a pH down solution needs to be easy to administer, cheap and provide some increase in buffering capacity overtime – to make additions less frequent – so the pH down product or combination of products that best fits this bill will depend on which of the above characteristics is more important for each particular user.
People who use drain-to-waste systems usually go for stronger acids, since they only adjust pH once before watering and then forget about the solution. This means that additional buffering capacity in the solution is probably not going to be very important and cost is likely the most important driving factor. If injectors are used then the strong acids are often diluted to the concentration that makes the most sense for them and most commonly either phosphoric or sulfuric acids are used.
For growers in recirculating systems options that adjust pH with some added buffering capacity are often preferred, because the same solution is constantly subjected to interactions with the plants. In this case it’s usually preferred to create a mixture of strong and weak buffering agents so that both quick decreases in pH and some increased protection from further increases can be given to the solution. In automated control systems using something like a concentrated MKP solution is preferable over any sort of solution containing phosphoric acid, as issues from control failures are less likely to be catastrophic.


