isthis2012
Member
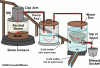
My dumb question of the day....what happens if you exhaust your hot air into a rubbermaid container filled with water? I honestly should have payed attention in grade 9 science, I know, I'm sorry. But treat my stupidity with kindness