After I switched from GH Flora 3-part to Grow More Sea Grow, I wondered the actual NPK ratios (and strength) had been at the various "mixes" of GH Flora, and whether that could be duplicated with my Grow More nutrients. I created a spreadsheet (<<link) and thought this might be useful to others.
For example, if someone uses one of the more boutique nutrient lines, this spreadsheet could help them see the details of what they feed and try other products mixed to similar proportions.
Here's an example from the README:
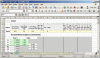
In the UNMIX worksheet enter the quantities (ml volume of liquid product, or grams weight of dry) you used for a feeding. See the yellow cells:
Those are half-strength of GH's recommended “1-2-3” ratio and Koolbloom powder at recommended strength. The results in the green cells are “Parts” (ratio) of product weights.
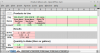
Enter those parts into the MIX worksheet's “Parts” row and you will see the NPK of those mixed products:
Next, scroll down to the “Strength” row and enter the total grams (from the UNMIX worksheet) and you'll see the PPM produced by those combined products at that strength. Notice that it tells you how much of each product to use (in milliliters or grams if dry). These quantities should be exactly the same as what you entered in the MIX worksheet.
Now you can play with the “Parts” of other nutrients to approximate that NPK. In this example I'll use Grow More Sea Grow All Purpose (veg), Flower & Bloom, Hawaiian Bud (booster) and Gen. Organic's CaMg+. The following “Parts” produce an NPK pretty similar:
Next adjust the “Strength” to get the desired PPM:
The resulting amounts to use are in a reasonable range. For the dry products, that's about 1/3 tsp, 2/3 tsp and 1/8 tsp respectively. CaMg+ is 4-3/4 tsp. These are within the manufacturer's suggested quantities.
Note: You'll need to weigh a tablespoon (or cup, 16 Tbsp) of your dry nutrients to know how results in grams relate to a volume in teaspoons.
You can adjust the “Volume: Quantity to make” and the spreadsheet will tell you how much of each component to use to make that NPK ratio, PPM strength for a quantity of water.
I put the spreadsheet on my blog page (<<link) so I can update it. I'm wondering if others see any serious flaws in what I'm doing. I created it in OpenOffice. It should work in Excel, but I don't have it. If it doesn't work, download OpenOffice here. The spreadsheet uses ordinary formulas. (No macros.).
For me it's been kind of fun. For example, seeing how Botanicare's CalMag+ adds N to my flowering ratio. I'm trying General Organic's CaMg+ which has no N (thanks to Mick941 for suggesting that.).
We often hear a flower ratio of 1-3-2 is the best. Or, that Advanced Nutrients maintains a 2-1 PK ratio. It's been fun to me to see how I can mix 2-3 Grow More products to achieve similar ratios.
Thoughts?
For example, if someone uses one of the more boutique nutrient lines, this spreadsheet could help them see the details of what they feed and try other products mixed to similar proportions.
Here's an example from the README:
=> Begin <=
You use GH Flora Series (3-part) and Koolbloom (liquid and powder “bloom booster”). You'd like to try a new product. You wonder how “different” it is, or how to achieve similar NPK ratios .In the UNMIX worksheet enter the quantities (ml volume of liquid product, or grams weight of dry) you used for a feeding. See the yellow cells:
Those are half-strength of GH's recommended “1-2-3” ratio and Koolbloom powder at recommended strength. The results in the green cells are “Parts” (ratio) of product weights.
Enter those parts into the MIX worksheet's “Parts” row and you will see the NPK of those mixed products:
Next, scroll down to the “Strength” row and enter the total grams (from the UNMIX worksheet) and you'll see the PPM produced by those combined products at that strength. Notice that it tells you how much of each product to use (in milliliters or grams if dry). These quantities should be exactly the same as what you entered in the MIX worksheet.
Now you can play with the “Parts” of other nutrients to approximate that NPK. In this example I'll use Grow More Sea Grow All Purpose (veg), Flower & Bloom, Hawaiian Bud (booster) and Gen. Organic's CaMg+. The following “Parts” produce an NPK pretty similar:
Next adjust the “Strength” to get the desired PPM:
The resulting amounts to use are in a reasonable range. For the dry products, that's about 1/3 tsp, 2/3 tsp and 1/8 tsp respectively. CaMg+ is 4-3/4 tsp. These are within the manufacturer's suggested quantities.
Note: You'll need to weigh a tablespoon (or cup, 16 Tbsp) of your dry nutrients to know how results in grams relate to a volume in teaspoons.
You can adjust the “Volume: Quantity to make” and the spreadsheet will tell you how much of each component to use to make that NPK ratio, PPM strength for a quantity of water.
=> End <=
I put the spreadsheet on my blog page (<<link) so I can update it. I'm wondering if others see any serious flaws in what I'm doing. I created it in OpenOffice. It should work in Excel, but I don't have it. If it doesn't work, download OpenOffice here. The spreadsheet uses ordinary formulas. (No macros.).
For me it's been kind of fun. For example, seeing how Botanicare's CalMag+ adds N to my flowering ratio. I'm trying General Organic's CaMg+ which has no N (thanks to Mick941 for suggesting that.).
We often hear a flower ratio of 1-3-2 is the best. Or, that Advanced Nutrients maintains a 2-1 PK ratio. It's been fun to me to see how I can mix 2-3 Grow More products to achieve similar ratios.
Thoughts?
Last edited: