LurchLurkin
Active Member
Ok so this formulations is my own but feel free to use it. This makes a 1 gallon at 200x concentrate so adjust to your desired EC. It is based somewhat off of Fatmans nutrients. The program I used was hydrobuddy and the PPM may seem a little bit off especially in calcium since I based my calculations off my water which was something like 58ppm Ca and 14ppm Mg or so if I remember and maybe 4-20 S. Clearly the EC is super high too but that may also be based off my water in part but I suggest just adding and dosing as you would any other fertilizer i.e. ~300ppm for seedlings up to ~1000ppm for mature
You could also have a brewers(as in beer brewing) test done of your water and download hydrobuddy for free and just put in the desired ppm and chemicals I've listed to see what you would come up with.
This is closer to 3-1-4 and designed for use in aeroponics with a tighter spacing in a SCROG, higher temps low humidity and high CO2. Fatman used a ~320ppm N for veg and ~285ppm N for flowering but I think either recipe could be used all the way through.
Note: the recipe contains a small amount of ammonium which is not directly useable by the plant. This can only be used if you have nitrogen fixing bacteria present which I do not recommend in a hydro grow. These bacteria are only beneficial when there is NH4 available while you are lacking in NO3 and can be detrimental to plant growth if enough NO3 is provided.
With that in mind, it is difficult to get a higher nitrogen content without raising your calcium super high and then needing to add more magnesium to maintain the 2:1 Ca:Mg ratio.
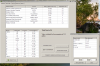
First Recipe:
Values calculated for the preparation of 1 gallons
Soln - Chemical Name Chemical Formula Grams
A - Potassium Nitrate KNO3 515.153
B - Manganese Sulfate (Monohydrate) MnSO4.H2O 11.646
B - Boric Acid H3BO3 21.653
B - Copper Sulfate (pentahydrate) CuSO4.5H2O 2.975
B - Sodium Molybdate (Dihydrate) Na2MoO4.2H2O 0.172
A - Iron DTPA FeDTPA 106.1
B - Magnesium Sulfate [Epsom Salt](Heptahydrate) MgSO4.7H2O 1013.538
B - Potassium Monobasic Phosphate KH2PO4 273.637
B - Zinc Sulfate (Heptahydrate) ZnSO4.7H2O 16.646
A - Yara Calcium Nitrate Yara_Ca(NO3)2 1021.991
PPM
N (NO3-) 288.669
K 371.976
P 82.256
Mg 150
Ca 314.482
S 186.045
Fe 10
Zn 5
B 5
Cu 1
Mo 0.09
Na 21.043
Si 0
Cl 3
Mn 5
N (NH4+) 14.849
EC=3.2 mS/cm
I recommend supplementing with Si but it is incompatible with the nutrient mixes so it would need to be added on its own. I like Botanicare because they use basically a 1mL-1L formula recommendation except I would recommend it all the way through the grow with each weekly res change as opposed to their recommendation of just through flower. It's used to strengthen the cell walls and that's def best done during vegetative growth too.
AN sells a supplement but they want to use like 2mL-1L and it's way more expensive. They're both just mixtures of sodium silicate and potassium silicate and I think some Potash or something so they will raise the pH.
You could also have a brewers(as in beer brewing) test done of your water and download hydrobuddy for free and just put in the desired ppm and chemicals I've listed to see what you would come up with.
This is closer to 3-1-4 and designed for use in aeroponics with a tighter spacing in a SCROG, higher temps low humidity and high CO2. Fatman used a ~320ppm N for veg and ~285ppm N for flowering but I think either recipe could be used all the way through.
Note: the recipe contains a small amount of ammonium which is not directly useable by the plant. This can only be used if you have nitrogen fixing bacteria present which I do not recommend in a hydro grow. These bacteria are only beneficial when there is NH4 available while you are lacking in NO3 and can be detrimental to plant growth if enough NO3 is provided.
With that in mind, it is difficult to get a higher nitrogen content without raising your calcium super high and then needing to add more magnesium to maintain the 2:1 Ca:Mg ratio.
First Recipe:
Values calculated for the preparation of 1 gallons
Soln - Chemical Name Chemical Formula Grams
A - Potassium Nitrate KNO3 515.153
B - Manganese Sulfate (Monohydrate) MnSO4.H2O 11.646
B - Boric Acid H3BO3 21.653
B - Copper Sulfate (pentahydrate) CuSO4.5H2O 2.975
B - Sodium Molybdate (Dihydrate) Na2MoO4.2H2O 0.172
A - Iron DTPA FeDTPA 106.1
B - Magnesium Sulfate [Epsom Salt](Heptahydrate) MgSO4.7H2O 1013.538
B - Potassium Monobasic Phosphate KH2PO4 273.637
B - Zinc Sulfate (Heptahydrate) ZnSO4.7H2O 16.646
A - Yara Calcium Nitrate Yara_Ca(NO3)2 1021.991
PPM
N (NO3-) 288.669
K 371.976
P 82.256
Mg 150
Ca 314.482
S 186.045
Fe 10
Zn 5
B 5
Cu 1
Mo 0.09
Na 21.043
Si 0
Cl 3
Mn 5
N (NH4+) 14.849
EC=3.2 mS/cm
I recommend supplementing with Si but it is incompatible with the nutrient mixes so it would need to be added on its own. I like Botanicare because they use basically a 1mL-1L formula recommendation except I would recommend it all the way through the grow with each weekly res change as opposed to their recommendation of just through flower. It's used to strengthen the cell walls and that's def best done during vegetative growth too.
AN sells a supplement but they want to use like 2mL-1L and it's way more expensive. They're both just mixtures of sodium silicate and potassium silicate and I think some Potash or something so they will raise the pH.