Porky101
Well-Known Member
Hi everyone,
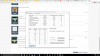
I have found a farming company that offer the following products:
Please can someone help me make a nice mix for VEG and then another for bloom.
The best however would be if someone explained in more detail what each chemical is for so I will beable to make my own mixes too.
Thanks guys!
AMM003 Fer Ammonium Sulphate (A) 50kg 50kg
MAS001 Fer Calcium Nitrate 25kg (P) 25kg
CAL012 Fer Calmabon 25kg
PRO001 Fer Calmabon Plus 20lt
COP003 Fer Copper Sulphate 25kg 25kg
HYG003 Fer Hydroponic 25kg 25kg
MAG002 Fer Magnesium Nitrate 25kg 25kg
MAG010 Fer Magnesium Sulphate (A) 25kg 25kg
MAN006 Fer Manganese Sulphate 25kg 25kg
MAP001 Fer MAP 25kg 25kg
MKP007 Fer MKP (A) 25kg 25kg
MUL001 Fer Multifeed 25kg 25kg
POT016 Fer Potassium Carbonate 25kg 25kg
KCL001 Fer Potassium Chloride (KCl) 25kg 25kg
POT013 Fer Potassium Nitrate (A) 25kg 25kg
SOL002 Fer Potassium Sulphate (A) 25kg 25kg
SUP001 Fer Supafeed (25kg) 25kg
SCH00 Fer Ultrabor (25kg) 25kg
URE001 Fer Urea LB (O) 25kg 25kg
ZIN006 Fer Zinc Sulphate Monohydrate 25kg 25kg
I have found a farming company that offer the following products:
Please can someone help me make a nice mix for VEG and then another for bloom.
The best however would be if someone explained in more detail what each chemical is for so I will beable to make my own mixes too.
Thanks guys!
AMM003 Fer Ammonium Sulphate (A) 50kg 50kg
MAS001 Fer Calcium Nitrate 25kg (P) 25kg
CAL012 Fer Calmabon 25kg
PRO001 Fer Calmabon Plus 20lt
COP003 Fer Copper Sulphate 25kg 25kg
HYG003 Fer Hydroponic 25kg 25kg
MAG002 Fer Magnesium Nitrate 25kg 25kg
MAG010 Fer Magnesium Sulphate (A) 25kg 25kg
MAN006 Fer Manganese Sulphate 25kg 25kg
MAP001 Fer MAP 25kg 25kg
MKP007 Fer MKP (A) 25kg 25kg
MUL001 Fer Multifeed 25kg 25kg
POT016 Fer Potassium Carbonate 25kg 25kg
KCL001 Fer Potassium Chloride (KCl) 25kg 25kg
POT013 Fer Potassium Nitrate (A) 25kg 25kg
SOL002 Fer Potassium Sulphate (A) 25kg 25kg
SUP001 Fer Supafeed (25kg) 25kg
SCH00 Fer Ultrabor (25kg) 25kg
URE001 Fer Urea LB (O) 25kg 25kg
ZIN006 Fer Zinc Sulphate Monohydrate 25kg 25kg