Interesting about keeping it subzero temp. Hadn't heard that before. To get the cleanest extract though, it might be made with water at about 325-350 f in an enclosed batch reactor. At those temperatures water has similar polarity to alcohol and you actually get higher yields. It's called subcritical water extraction. The pressure from being enclosed keeps it liquid.
I guess you'd have to leach the material with water at normal pressure first so you won't pick up that crud with the pressurized water later, unless you had a way to only have the pressurized heated water ever come in contact with the material. The apparatus is usually constructed so that the extraction vessel and much of the piping is inside an oven of sorts. Temps used are usually 150-200 C, I said 325-350 f earlier, which is about 160-180 C. Obviously water is cheaper and easier to get than other solvents, including CO2. It's also completely non-toxic and environmentally friendly. Just need to use stainless steel pipe for construction. Actually, BHO apparatus may be modifiable, if it's thick enough.
Here's a
patent with info on subcritical water.
Good score brother BC! Sounds like a fun project worthy of further investigation!
Here is a steam chest table, which shows that at 200C, pressure is only about 420 psi.
http://www.turnkeyips.com/assets/steam_temperature_pressure_table.pdf
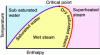
Attached is a water saturation curve:
My personal experience designing for super heated steam, is limited to a super heater return tube and hydrostatically pressure testing the systems for the US Bicentenial Freedom train 4449 Daylighter restoration in 1976, so would have to refresh my memory to be certain there aren't special rules for super heated steam, but in general ASME Section IX boiler code would require a pressure vessel capable of withstanding 3X the maximum operating pressure and have a pressure relief at about 125% of that pressure.
(Any steam buffs check:
http://www.freedomtrain.org/american-freedom-train-home.htm)
1260 psi is not hard to contain in a tube, because the stainless is in tensile, but the end caps become an issue due to deflection, where they attempt to deform into a hemisphere, so the end caps have to be significantly heavier (more beam/depth), to resist it, or start as a hemisphere already.
ASME considers 6" diameter and below piping and above a pressure vessel. Pressure piping rules are easier, because stresses are less, so you will note that SCFE CO2 extraction columns are typically 6".
6" Schedule 40 stainless pipe is rated at 1219 psi, cutting the margin thin, but Schedule 80 is rated for 1913 psi, so would easily work with ANSI flanges and plumbed blind end caps, or ASME dished heads.
http://www.engineeringtoolbox.com/stainless-steel-pipes-pressure-ratings-d_346.html
We used Sprague pneumatic intensifier pumps to pump water at higher pressure, and 420 psi is well within their capabilities on shop air. That is where I would start searching.