Sasha's Words on this
One of the banes of the archivist is having to choose one pattern of organization over another. The book store owned by a language scholar will have the German poets and playwrights and novelists here, and the French ones over there. Next door, the book store is run by a letters scholar, and the poetry of the world is here, and the plays of the world are there, regardless of the language of origin. The same obtains with spices, and essential oils, and amphetamines. The spice cabinet is a rich source of chemical treasures, each source plant containing a host of com-pounds, some of which are true essential oils. And the next spice from the next plant has some of the same components and some new ones. Does one organize by plant (spice or herb) or by essential oil (amphetamine)? Let's do it by the ring substitution pattern of the amphetamine, and gather the spices and oils as a secondary collection.
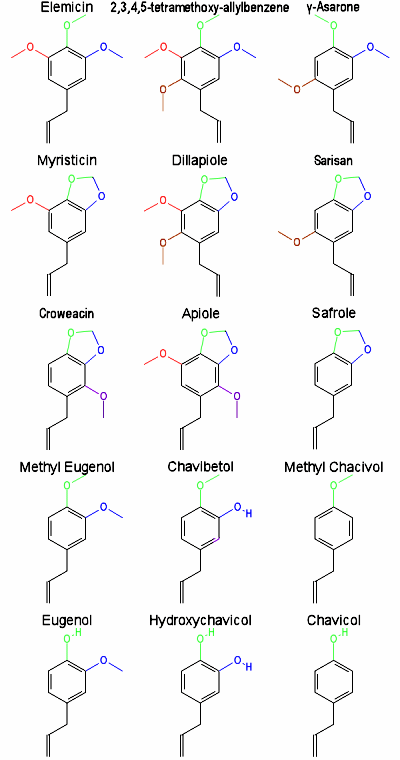
(1) The 4-methoxy pattern. The pivotal essential oil is 4-allylanisole, or methyl chavicol, or estragole (called esdragol in the old literature). This allyl compound is found in turpentine, anise, fennel, bay, tarragon, and basil. Its smell is light, and reminiscent of fennel. The propenyl analogue is called anethole, or anise camphor, and it is found in both anise and camphor. It is a waxy solid, and has a very intense smell of anise or fennel. At low concentrations, it is sweet, as in magnolia blossoms, where it is also found. The drinks that turn cloudy with water dilution (Pernod-like liqueurs, and ouzo and roki), are heavy with it, since it was the natural flavoring in the original absinthe. That drink was very popular in the last century, as an intoxicant which produced an altered state of consciousness beyond that which could be ascribed to alcohol alone. It contained wormwood, which proved to be neurologically damaging. The flavorings, such as anethole, are still big things in synthetic liqueurs such as vermouth. Old anethole, when exposed to air and light, gets thick and sticky and yellowish, and becomes quite disagreeable to taste. Maybe it is polymerizing, or maybe oxidizing to stuff that dimerizes. Whatever. These changes are why old spices in the cabinet are best discarded. And adding ammonia to any of these natural product oils produces, in principle, 4-methoxyamphetamine, 4-MA.
(2) The 3,4-dimethoxy pattern. The main actor here is methyleugenol, or 4-allyl-1,2-dimethoxybenzene. This is located in almost every item in the spice cabinet. It is in citronella, bay (which is laurel, which is myrtle), pimiento, allspice, pepper, tree-tea oil, and on and on. It has a faint smell of cloves, and when dilute is immediately mistaken for carnations. The propenyl analogue is, not unreasonably, methylisoeugenol, a bit more scarce, and seems to always be that little minor peak in any essential oil analysis. The compounds missing that methyl group on the 4-oxygen are famous. The allyl material is eugenol, 4-allylguaiacol, and it is in cinnamon, nutmeg, cloves, sassafras and myrrh. You taste it and it burns. You smell it and think immediately of cloves. And its property as an anesthetic, in the form of a clove, is well known in the folk-treatment of toothaches. Actually, flowers of clove (the gillyflower, like the carnation) are the small, pointy things that decorate baked hams and, when stuck into apples, make pomander balls. This anesthetic property has recently led to a drug abuse fad, called clove cigarettes. Very strong, very flavorful, and very corrosive things from Southeast Asia. The eugenol that is present numbs the throat, and allows many strong cigarettes to be smoked without pain. The propenyl analogue is isoeugenol, with a smell that is subtle but very long lasting, used more in soaps and perfumes than in foods. The amine addition to the methyleugenol world produces 3,4-dimethoxyamphetamine, or 3,4-DMA. The isomer with the other methyl group missing is chavibetol (3-hydroxy-4-methoxyallylbenzene) and is found in the pepper leaf that is used with betel nut. A couple of positional rearrangement isomers of methyleugenol are known in the plant world. The 2,4-isomer is called osmorrhizole, and the conjugated form is isoosmorrhizole or nothosmyrnol; both are found in carrot-like vegetables. They, with ammonia, would give 2,4-DMA. And the 3,5-dimethoxyallylbenzene isomer from artemisia (a pungent herb commonly called mugwort) and from sage, would give rise to 3,5-DMA. This is an unexplored isomer which would be both an antidote for opium as well as a stimulant, if the classical reputation of mugwort is transferred to the amphetamine.
(3) The 3,4-methylenedioxy pattern. One of the most famous essential oils is safrole, or 4-allyl-1,2-methylenedioxybenzene. This is the mainstay of sassafras oil, and it and its conjugated isomer isosafrole have a smell that is immediately familiar: root beer! These are among the most widely distributed essential oils, being present in most of the spices, including the heavies such as cinnamon and nutmeg. I am not aware of the 2,3-isomer ever having been found in nature. Adding ammonia to either would give MDA.
(4) The 3-methoxy-4,5-methylenedioxy pattern. The parent compound is myristicin, 5-allyl-1-methoxy-2,3-methylenedioxybenzene, and the source of this is nutmeg (or the botanically parallel material, mace). The nutmeg is the seed of the tree Myristica fragrans and mace is the fibrous covering of the seed. The two spices are virtually identical as to their chemical composition. Myristicin and the conjugated isomer isomyristicin are also found in parsley oil, and in dill. This was the oil that was actually shown to be converted to MMDA by the addition of ammonia by passage through an in vitro liver preparation. So here is the major justification for the equation between the essential oils and the Essential Amphetamines. Care must be taken to make an exact distinction between myristicin (this essential oil) and myristin (the fat) which is really trimyristin or glyceryl trimyristate from nutmeg and coconut. This is the fat from myristic acid, the C-14 fatty acid, and these two similar names are often interchanged even in the scientific literature.
(5) The 2-methoxy-3,4-methylenedioxy pattern. This is the second of the three natural methoxy methylenedioxy orientations. Croweacin is 2-methoxy-3,4-methylenedioxyallylbenzene, and it takes its name from the binomial for the plant Eriostemon crowei from the worlds of rue and the citrus plants. It corresponds to the essential amphetamine MMDA-3a. This oil is found in plants of the Family Rutaceae. My memories of this area of botany are of Ruta graveolens, the common rue, whose small leaves smelled to me, for all the world, like cat urine. This plant has always fascinated me because of a most remarkable recipe that I was given by a very, very conservative fellow-club member, one evening, after rehearsal. He told me of a formula that had provided him with the most complete relief from arthritic pain he had ever known. It was a native decoction he had learned of many years eariler, when he was traveling in Mexico. One took equal quantities of three plants, Ruta graveolens (or our common rue), Rosmarinus officinalis (better known as rosemary), and Cannabis sativa (which is recognized in many households simply as marijuana). Three plants all known in folklore, rue as a symbol for repentance, rosemary as a symbol of remembrance, and pot, well, I guess it is a symbol of a lot of things to a lot of people. Anyway, equal quantities of these three plants are allowed to soak in a large quantity of rubbing alcohol for a few weeks. Then the alcoholic extracts are clarified, and allowed to evaporate in the open air to a thick sludge. This then was rubbed on the skin, where the arthritis was troublesome, and always rubbed in the direction of the extremity. It was not into, but onto the body that it was applied. All this from a very conservative Republican friend!
The methoxy-methylenedioxy pattern is also found in nature with the 2,4,5-orientation pattern. The allyl-2,4,5-isomer is called asaricin. It, and its propenyl-isomer, carpacin, are from the Carpano tree which grows in the Solomon Islands. All these plants are used in folk medicine. These two systems, the 2,3,4- and the 2,4,5-orientations, potentially give rise, with ammonia, to MMDA-3a and MMDA-2.