Jimdamick
Well-Known Member
Always been taught 5.8 is the sweet spot if you use water, but 6.2-6.5 PH for dirt. Why, seeing as the most balanced uptake for all nutes and minerals, at least for Cannabis, is actually 6.0-6.1 I'm confused, so please enlighten me. I'll bet no one knows, and that's why all my food is at 6.0, because I'm a dickhead (no surprise for anyone that knows me)
+REP for good reasons.
+REP for good reasons.
Last edited:

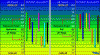
 I would assume in searching for the most suitable ph for the absorption and use of macro and micro nutrients in cannabis there would have to be strenuous tests testing the exact soil composition and how they displace electrons and bond given certain conditions. Given the same could be assumed in hydro from how the photons affect your reservoir, if there are other elements in your water, possible leeching from plastic or pumps, etc. But I'm with hotrod on this one, think it's just a general theory(consensus) that somewheres between 6.2-6.8 has best absorption for micros and macros in soil and 5.5-6 for hydro. IMO.
I would assume in searching for the most suitable ph for the absorption and use of macro and micro nutrients in cannabis there would have to be strenuous tests testing the exact soil composition and how they displace electrons and bond given certain conditions. Given the same could be assumed in hydro from how the photons affect your reservoir, if there are other elements in your water, possible leeching from plastic or pumps, etc. But I'm with hotrod on this one, think it's just a general theory(consensus) that somewheres between 6.2-6.8 has best absorption for micros and macros in soil and 5.5-6 for hydro. IMO.