Crazy pH Swings – How Media and Bacteria Affect pH in Hydroponics
Not my words...
May 21st, 2010 | Author: admin
I usually get an email from time to time from someone who is experiencing wild pH swings in their hydroponic reservoirs. Growers usually tell me that their pH was around 6.2 one day and then 8.0 by the next morning or some similar story. This situation becomes a little bit frustrating as the grower does a huge effort to keep the solution at a certain pH level only to realize that after a certain time the pH of the solution simply starts to swing wildly between very odd values. In order to help new and experienced growers better understand the nature of these swings, what they mean, and how they can be eliminated for good I decided to write this small article on hydroponic pH swings and how variables different to plant feeding affect pH levels. Let’s suppose you got home from work, prepared a new batch of nutrient solution and set your pH level at a very comfortable level of 5.7. By the next day, when you wake up in the morning to check your plants you find that the pH of your solution is 7.5. You start to argue with your pH meter, recalibrate, readjust your solution and leave for work. When you come back – to your surprise – your pH level is now around 7.3. What ? – you ask yourself – What could be wrong if you set the pH to 5.7 again and it again went up to 7.3 ?
--
The answer to these wild pH swings comes from an understanding of the chemistry behind everything within your hydroponic system. Generally these swings towards high pH values are caused by media which has surface-active basic sites which act like “buffers” and readjust the pH of your nutrient solution to their own “preset” pH level. This is very much like the mechanism used by soils to naturally control pH, only that this time the minerals are playing against you. Substrates that have been made at high temperatures which have basic potential – such as rockwool – show this kind of behavior. Other media such as river bed gravel also show strong pH buffering effects due to their natural mineral composition.
How do you end this problem ? The easiest way to end this problem is to pretreat your media before starting your crop. Place your media in a bucket and then add 1 liter of vinegar for every gallon of water. The media will attempt to neutralize the acetic acid and in doing so it will lose the proton capturing ability of its surface basic sites. Using a weak acid like acetic acid is better than using a strong acid – like nitric acid – because this ensures that residual acids within the media won’t lead to other extreme pH fluctuations. After the media is soaked in the vinegar solution measure the pH, wait a day and measure it again. If there is no difference between both readings then you can now wash and use the media – if there is – then you need to wait another day and remeasure.
Now basic media is not the only problem around. There are also wild swings to acid values which are usually a consequence of bacterial growth or dying organic matter. When organisms die or when they are being decomposed by bacteria organic acids – which lower pH – are released into your nutrient solution. Wild swings into the 3.5-4.5 region usually mean that the problem is not media but related to root disease. You should do a hydrogen peroxide treatment (check my articles on peroxide for more on this) and wait to see if pH levels stabilize after a while. In extreme cases, physical removal of dead root material may be necessary to correct the problem.
Last but not least, the problem can also be related with plant feeding from a very scarse volume of solution. If you are handling less than 1 gallon per plant of solution in your reservoir then it is likely that plants themselves – through the absorption of nutrients – are causing the swings. This is easily fixed by placing a larger reservoir and ensuring that you are always recirculating at least 1 gallon per plant of nutrient solution. Hopefully with the above guide you will be able to better understand “wild” pH swings and take corrective action whenver you see this behavior happening within your hydroponic crop.
PH Electrode Care and Maintenance...
<b>Understanding why your PH does what it does...
Not my words...
May 21st, 2010 | Author: admin
I usually get an email from time to time from someone who is experiencing wild pH swings in their hydroponic reservoirs. Growers usually tell me that their pH was around 6.2 one day and then 8.0 by the next morning or some similar story. This situation becomes a little bit frustrating as the grower does a huge effort to keep the solution at a certain pH level only to realize that after a certain time the pH of the solution simply starts to swing wildly between very odd values. In order to help new and experienced growers better understand the nature of these swings, what they mean, and how they can be eliminated for good I decided to write this small article on hydroponic pH swings and how variables different to plant feeding affect pH levels. Let’s suppose you got home from work, prepared a new batch of nutrient solution and set your pH level at a very comfortable level of 5.7. By the next day, when you wake up in the morning to check your plants you find that the pH of your solution is 7.5. You start to argue with your pH meter, recalibrate, readjust your solution and leave for work. When you come back – to your surprise – your pH level is now around 7.3. What ? – you ask yourself – What could be wrong if you set the pH to 5.7 again and it again went up to 7.3 ?
--
The answer to these wild pH swings comes from an understanding of the chemistry behind everything within your hydroponic system. Generally these swings towards high pH values are caused by media which has surface-active basic sites which act like “buffers” and readjust the pH of your nutrient solution to their own “preset” pH level. This is very much like the mechanism used by soils to naturally control pH, only that this time the minerals are playing against you. Substrates that have been made at high temperatures which have basic potential – such as rockwool – show this kind of behavior. Other media such as river bed gravel also show strong pH buffering effects due to their natural mineral composition.
How do you end this problem ? The easiest way to end this problem is to pretreat your media before starting your crop. Place your media in a bucket and then add 1 liter of vinegar for every gallon of water. The media will attempt to neutralize the acetic acid and in doing so it will lose the proton capturing ability of its surface basic sites. Using a weak acid like acetic acid is better than using a strong acid – like nitric acid – because this ensures that residual acids within the media won’t lead to other extreme pH fluctuations. After the media is soaked in the vinegar solution measure the pH, wait a day and measure it again. If there is no difference between both readings then you can now wash and use the media – if there is – then you need to wait another day and remeasure.
Now basic media is not the only problem around. There are also wild swings to acid values which are usually a consequence of bacterial growth or dying organic matter. When organisms die or when they are being decomposed by bacteria organic acids – which lower pH – are released into your nutrient solution. Wild swings into the 3.5-4.5 region usually mean that the problem is not media but related to root disease. You should do a hydrogen peroxide treatment (check my articles on peroxide for more on this) and wait to see if pH levels stabilize after a while. In extreme cases, physical removal of dead root material may be necessary to correct the problem.
Last but not least, the problem can also be related with plant feeding from a very scarse volume of solution. If you are handling less than 1 gallon per plant of solution in your reservoir then it is likely that plants themselves – through the absorption of nutrients – are causing the swings. This is easily fixed by placing a larger reservoir and ensuring that you are always recirculating at least 1 gallon per plant of nutrient solution. Hopefully with the above guide you will be able to better understand “wild” pH swings and take corrective action whenver you see this behavior happening within your hydroponic crop.
PH Electrode Care and Maintenance...
PH Electrode Care and MaintenanceYour pH electrode is the most sensitive component of your pH instrument. Correct calibration procedures combined with proper maintenance will provide years of reliable measurement.
Calibration
Since glass pH electrodes measure H+ (hydrogen ion) concentration relative to their reference half-cells, they must be calibrated periodically to ensure accurate, repeatable measurements. Our wide selection of commercial pH calibration buffer sachets include solutions that are standardized against NIST-certified pH references for calibrating meters with resolution up to 0.01pH.
Although calibration against one pH reference buffer (one-point calibration) typically ensures accurate pH measurement, frequent two-point or even three-point calibrations ensure the most reliable results. Make sure your pH system includes calibration buffers for a range of pH values.
Handling
During shipment it is possible for air bubbles to move into the glass bulb. To remove air bubbles, shake down the electrode in the same manner as a clinical thermometer until the glass bulb is filled with solution.
Rinse electrodes with distilled water before and after measuring a sample. Blot the end of the electrode with lint-free paper to remove excess water. NOTE: Never wipe the electrode to remove excess water - wiping can create static charges that interfere with correct pH measurement.
Conditioning
After removing the electrode from soaking bottle or protective cap at the bottom of sensor, place the electrode in a clean container containing one of the liquids i.e. 4.0 M KCl or pH 7.0 buffer. Soak electrode for 30 minutes if left dry. NOTE: Never condition the electrode in distilled water or deionised water - long term exposure to pure water will damage the special glass membrane.
After conditioning the sensor, rinse the electrode with distilled or deionised water. The electrode is ready for calibration and measurement.
Storing
The sensor should never be stored dry. Always keep pH electrode moist. Proper pH electrode storage maximizes electrode performance and extends electrode life. It is best to store electrodes in clean containers filled with pH storage solution, EC-RE005. Do not store an electrode in distilled or deionised water - this will cause ions to leach out of the glass bulb and render your electrode useless.
Cleaning
The solution used to clean pH electrode depends on the presence of possible contaminants. Mechanically intact electrodes may show slow response due to coating or clogging. Use the guide below to choose the appropriate cleaning solution options:-
After any of the cleaning procedures, it is good practice to thoroughly rinse the pH electrode with deionised water, drain and refill the reference chamber if necessary before use.
Reservoir Maintenance
The routine task of keeping the hydroponic nutrient solution in the reservoir from becoming too strong or toxic as the water is being evaporated and the nutrients within the solution are taken up by the plants.
Simply put...
Top off daily with *half strength nutrients, alternating days topping up with plain water. Change the entire reservoir with fresh solution every ten days to two weeks. (*half the strength of your current new reservoir starting strength)
Why should you?
One problem in hydroponics solution maintenance, as water is being taken up by the plants as well as evaporating out of the solution, the concentration of nutrient salts in the solution becomes gradually stronger, sometimes to the point of certain elements becoming toxic to the plants. The TDS will always become stronger as water is taken away from the solution.
Another problem, is that hydroponically grown plants will take up what they need as they need it from the nute solution. A nutrient solution left alone will end up lacking key nutrients, with a build-up of *toxic levels of other key nutrients. *Toxic in the solution, as well as in the plants.
The only way around these problems for the average hydroponic grower, is to practice sound reservoir topping off procedures. The most widely accepted maintenance method, involves daily topping off and routine reservoir solution replacement. IE: Topping off the reservoir daily with a nutrient solution which is half of the current new reservoir strength, alternating days by topping off with plain water and finally, changing the entire res solution at least every two weeks.
Changing the reservoir solution every two weeks, will give the plants a fresh and well balanced nute mix, which has not been altered by the plants nutrient uptake.
*Many scientific studies have been performed, which demonstrate these facts by GCMS testing of the nutrient solution contents and the nutrient salts contained within the actual plant tissues, as the plants "take-up" the specific nutrients in the solution.
Metaphorically speaking...
Plants will take up excessive levels of some nutrients, leaving the solution lacking in certain key nutrients. Just like a puppy would make him/her self sick if it were allowed to feed from a bottomless food bowl, plants grown hydroponically can harm themselves with nutrient deficiencies, lockouts and overdoses, if allowed to continue feeding without some control over whats available in the "food bowl".
Here's some more info on PH, not my words, but still worthy...Calibration
Since glass pH electrodes measure H+ (hydrogen ion) concentration relative to their reference half-cells, they must be calibrated periodically to ensure accurate, repeatable measurements. Our wide selection of commercial pH calibration buffer sachets include solutions that are standardized against NIST-certified pH references for calibrating meters with resolution up to 0.01pH.
Although calibration against one pH reference buffer (one-point calibration) typically ensures accurate pH measurement, frequent two-point or even three-point calibrations ensure the most reliable results. Make sure your pH system includes calibration buffers for a range of pH values.
Handling
During shipment it is possible for air bubbles to move into the glass bulb. To remove air bubbles, shake down the electrode in the same manner as a clinical thermometer until the glass bulb is filled with solution.
Rinse electrodes with distilled water before and after measuring a sample. Blot the end of the electrode with lint-free paper to remove excess water. NOTE: Never wipe the electrode to remove excess water - wiping can create static charges that interfere with correct pH measurement.
Conditioning
After removing the electrode from soaking bottle or protective cap at the bottom of sensor, place the electrode in a clean container containing one of the liquids i.e. 4.0 M KCl or pH 7.0 buffer. Soak electrode for 30 minutes if left dry. NOTE: Never condition the electrode in distilled water or deionised water - long term exposure to pure water will damage the special glass membrane.
After conditioning the sensor, rinse the electrode with distilled or deionised water. The electrode is ready for calibration and measurement.
Storing
The sensor should never be stored dry. Always keep pH electrode moist. Proper pH electrode storage maximizes electrode performance and extends electrode life. It is best to store electrodes in clean containers filled with pH storage solution, EC-RE005. Do not store an electrode in distilled or deionised water - this will cause ions to leach out of the glass bulb and render your electrode useless.
Cleaning
The solution used to clean pH electrode depends on the presence of possible contaminants. Mechanically intact electrodes may show slow response due to coating or clogging. Use the guide below to choose the appropriate cleaning solution options:-
- For general cleaning: Soak the pH electrode in 0.1 M Hydrochloric Acid or 0.1 M HNO3 for 20 minutes. Rinse well in tap water before use.
- For removing stubborn deposits and bacteria: Soak the pH electrode in a 1:10 dilution of household laundry bleach for 10 minutes. Rinse thoroughly before use.
- For removal of oil and grease: Rinse the pH electrode in mild detergent or methyl alcohol. Wash in water before use.
- For removal of protein deposits: Soak the pH electrode in 1% pepsin in 0.1m HCl (EC-DPC-BT) for 5 minutes. Rinse well in water before use.
After any of the cleaning procedures, it is good practice to thoroughly rinse the pH electrode with deionised water, drain and refill the reference chamber if necessary before use.
Reservoir Maintenance
The routine task of keeping the hydroponic nutrient solution in the reservoir from becoming too strong or toxic as the water is being evaporated and the nutrients within the solution are taken up by the plants.
Simply put...
Top off daily with *half strength nutrients, alternating days topping up with plain water. Change the entire reservoir with fresh solution every ten days to two weeks. (*half the strength of your current new reservoir starting strength)
Why should you?
One problem in hydroponics solution maintenance, as water is being taken up by the plants as well as evaporating out of the solution, the concentration of nutrient salts in the solution becomes gradually stronger, sometimes to the point of certain elements becoming toxic to the plants. The TDS will always become stronger as water is taken away from the solution.
Another problem, is that hydroponically grown plants will take up what they need as they need it from the nute solution. A nutrient solution left alone will end up lacking key nutrients, with a build-up of *toxic levels of other key nutrients. *Toxic in the solution, as well as in the plants.
The only way around these problems for the average hydroponic grower, is to practice sound reservoir topping off procedures. The most widely accepted maintenance method, involves daily topping off and routine reservoir solution replacement. IE: Topping off the reservoir daily with a nutrient solution which is half of the current new reservoir strength, alternating days by topping off with plain water and finally, changing the entire res solution at least every two weeks.
Changing the reservoir solution every two weeks, will give the plants a fresh and well balanced nute mix, which has not been altered by the plants nutrient uptake.
*Many scientific studies have been performed, which demonstrate these facts by GCMS testing of the nutrient solution contents and the nutrient salts contained within the actual plant tissues, as the plants "take-up" the specific nutrients in the solution.
Metaphorically speaking...
Plants will take up excessive levels of some nutrients, leaving the solution lacking in certain key nutrients. Just like a puppy would make him/her self sick if it were allowed to feed from a bottomless food bowl, plants grown hydroponically can harm themselves with nutrient deficiencies, lockouts and overdoses, if allowed to continue feeding without some control over whats available in the "food bowl".
<b>Understanding why your PH does what it does...
When water reacts with itself to create the H3O(+)(hydroxyl) and OH(-) (hydroxide) species, one of the most fundamental and important characteristics of aqueous solutions is generated. The reactivity of a solution and its interaction with living organisms is determined in a great extent by the concentration of these two species, a variable usually measured as pH which is nothing but the negative value of the logarithm of the concentration of the H3O(+) ion. In hydroponic culture – where our plants are in great contact with aqueous solutions – the understanding of the role of the H3O(+) and OH(-) ions and their measurement as pH becomes very important if an in-depth understanding of what is going on wants to be attained. On today’s post I will attempt to guide you into this micro world of pH and how and why pH changes within a hydroponic crop. What determines pH ? This variable is inversely proportional to the concentration of H3O(+) ions and directly proportional to OH(-) ions, the more hydroxil ions you have the more acidic your solution will be (the lower the pH) while more hydroxide ions will increase your pH and give you a higher pH reading. It is important to understand here that hydroxyl and hydroxide ions determine each other’s concentration. Since water’s self-reaction equilibria must be maintained, the sum of pH and pOH must always be equal to 14 (a neat consequence of chemical equilibrium theory). When the concentration of hydroxyl and hydroxide ions is equal, pH and pOH contribute equally to the solution and they are therefore both 7, reason why the pH of a neutral solution has this value. Now that we know a little bit about pH we can understand better what happens when plants interact with a nutrient solution. When a plant is put within a given solution it wants to absorb the nutrients it needs to grow. These nutrients are available as ions that have a given charge. For example, nitrogen is absorbed as the nitrate ion (NO3(-)) while potassium is absorbed as the K(+) ion. When a plant takes potassium in, it deplets the solution of a positive charge. Since the solution must remain neutral the plant gives the solution an H3O(+) ion to compensate. The plant has therefore decreased the pH of the solution by absorbing a potassium ion. When nitrate is absorbed – an ion with a negative charge- the plant does the opposite and exchanges the nitrate for an OH(-), the pH of the solution is increased.
-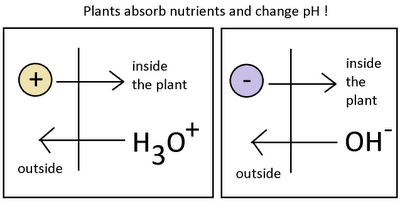 -
-
If plants absorbed nutrients in a perfectly symmetrical fashion, they would not increase or decrease pH as overall charge changes would be compensated. However – as no one is perfect- plants absorb nutrients at different rates and they therefore create a “pull” towards a certain pH region. If a plant absorbs nitrate heavily it will start to contribute far more OH(-) than H3O(+) ions into the solution and the result will be a net increase in pH. Depending on the composition of the nutrients and the overall growth stage of the plant, different net movements in pH can be achieved by the plant.
The most influential factor in the changes of pH within a solution is generally the composition of the nitrogen component of the solution. When plants absorb ammonium ions NH4(+) they tend to decrease pH while nitrate – as mentioned above – tends to increase pH when absorbed. If you contribute a percentage of the nitrogen in your solution as ammonia the net effect will be a beneficial “absorption pH buffer” since plants will take nitrogen in both forms, effectively delaying the onset of important pH variations. Of course, the ratio of nutrients also performs a vital role since plants’ nutrient absorption mechanism are largely non-specific and they are greatly influenced by the different concentrations of nutrients within the solutions. Having a nutrient solution designed to provide an adequate balance will be vital in helping you control pH fluctuations.
None of this is in my words, all of it was borrowed from different sources. Hope it helps someone new.
</b>-
 -
-If plants absorbed nutrients in a perfectly symmetrical fashion, they would not increase or decrease pH as overall charge changes would be compensated. However – as no one is perfect- plants absorb nutrients at different rates and they therefore create a “pull” towards a certain pH region. If a plant absorbs nitrate heavily it will start to contribute far more OH(-) than H3O(+) ions into the solution and the result will be a net increase in pH. Depending on the composition of the nutrients and the overall growth stage of the plant, different net movements in pH can be achieved by the plant.
The most influential factor in the changes of pH within a solution is generally the composition of the nitrogen component of the solution. When plants absorb ammonium ions NH4(+) they tend to decrease pH while nitrate – as mentioned above – tends to increase pH when absorbed. If you contribute a percentage of the nitrogen in your solution as ammonia the net effect will be a beneficial “absorption pH buffer” since plants will take nitrogen in both forms, effectively delaying the onset of important pH variations. Of course, the ratio of nutrients also performs a vital role since plants’ nutrient absorption mechanism are largely non-specific and they are greatly influenced by the different concentrations of nutrients within the solutions. Having a nutrient solution designed to provide an adequate balance will be vital in helping you control pH fluctuations.
None of this is in my words, all of it was borrowed from different sources. Hope it helps someone new.
