jpeg666
Well-Known Member
***I did not write this, I just want to share it! I think it is an absolute must read to understand what your are putting in your plants and what additives are BS and what ones are worth investing your hard earned money in***
Beneficial Additives in Hydroponics
Beneficial Additives in Hydroponics
I have previously stressed the point that plants will achieve optimum growth only after they have been provided with an ideal environment (temp, light, humidity etc) and ideal nutrition. I also stress the point that big yields do not come in bottles. That environment is king and a nutrient is only as good as the environment that it is used in.
That is not to say that some additives wont enhance the plants capacity to achieve optimum yields.
For instance, plant nutrition purchased in fluid or powder form does not typically (ever) provide all of the elements that are available to plants in nature. Just one example of this is the absence of silica in nutrient formulas. Silica is an extremely common element in nature but is not incorporated into nutrient formulas due to high pH and insolubility. This means that the average nutrient formula can be improved upon somewhat with the addition of silica.
There are also other elements found in nature that arent commonly found in nutrient formulas. Because of this we are able to fully provide for the plants nutritional needs with careful use of beneficial nutritional additives.
In addition to this, some additives can enhance naturally occurring aspects of plant growth. Other additives can control what the plant does. In other cases additives can aid in plant health, reducing the incidence of disease and pathogens. For instance, products such as beneficial microbes, or sterilizing agents, which well cover in depth in this chapter, are critical in any hydroponic system to ensure pathogens such as pythium and fusarium are controlled/prevented.
Anyway, lets have a closer look at what I consider to be some of the more important/valuable beneficial additives. Lets face it, with the vast array of additives on the market today, all growers have their own views with some using incredible amounts of additives and others using very few. Ultimately, each grower will make their own choices but for now lets have an in depth look at beneficial additive science and why it is that I consider several additives of great value to growers/yields.
Silicon (Si) and Hydroponics
Overview
Silicon (Si) is the second most common element on Earth after oxygen and is abundant in soils.
Siliconis abundant in all field grown plants, but it is not present in most hydroponicsolutions.
In plants, silicon strengthens cell walls, improving plant strength, health, and productivity.1
Silicon, deposited in cell walls of plants, has been found to improve heat and drought tolerance and increase resistance to insects and fungal infections. Silicon can help plants deal with toxic levels of manganese, iron, phosphorus and aluminium as well as zinc deficiency.
Thus, the beneficial effects of silicon (Si) are threefold: 1) it protects against insect and disease attack (Cherif et al. 1994; Winslow, 1992; Samuels, 1991), 2) it protects against toxicity of metals (Vlamis and Williams, 1967; Baylis et al. 1994), and 3) it benefits quality and yield of agricultural crops (Kathryn E Richmond et al, 2003).
Silica is excluded from hydroponic nutrient formulas because it has a high pH and is unable to remain soluble (hold/remain stable) in concentrated nutrient formulas.
Therefore, Si needs to be added to the nutrient tank as a separate element.
Benefits of Si
· Increased disease resistance
· Increased resistance to pathogenic airborne fungi (eg. Botrytis)
· Increased resistance to waterborne pathogens
· Increased resistance to insects/pests
· Increased strength in cell structure
· Increased stress tolerance
· Increased drought tolerance
· Increased salt tolerance
· Increased yields
THE SCIENCE
Silicon (Si) is not considered to be an essential plant nutrient because most plant species can complete their life cycle without it. However, Si is considered to be a beneficial element and some plant species can accumulate Si at concentrations higher than many essential macronutrients (Epstein, 1999).
A lack of knowledge about the role of silicon (Si) in horticultural crops became apparent with the change to soilless growing media (hydroponics) in the glasshouse industry in the Netherlands. It was found that in hydroponic systems the Si contents in plant tissue were significantly lower in comparison with crops grown in soil. Research was carried out on the effects of Si application in hydroponic systems. With cucumber, melon, courgette, strawberry, bean, and rose, the Si contents were increased as a result of the addition of Si into the root environment. Results in these trials showed that cucumber, rose, and courgette could benefit from enhanced Si concentration in the root environment, since total yield was increased and powdery mildew was suppressed. 2
Silica and Fungi Suppression (eg. Botrytis)
Si has been shown in numerous studies to suppress fungal pathogens such as Botrytis. In a study by Adatia et al (1986) conducted on cucumbers grown in recirculating hydroponic systems it was shown that despite regular applications of fungicide, outbreaks of the fungal disease occurred on most of the mature leaves of low Si cucumber plants, while the high Si plants remained almost completely free of fungal pathogens. The conclusion to this study noted:
The addition of Si could be beneficial to cucumbers grown in areas where the local water supply is low in this element, especially when grown in recirculating solution or in a medium low in Si, e.g. peat. 3
[End Quote]
Further research by Shettyet al (2011) demonstrated that Si treatment reduced powdery mildew development by inducing host defense responses in plants.4
It is believed that silicon deposition at sites of fungal pathogen penetration may be a common component of the host-defense response in a variety of plant families. 5
Silicon is also deposited in the cell walls of roots where it acts as a barrier against invasion by parasites and pathogens. 6
For instance, potassium silicate has been shown to act as a preventative against Pythium ultimum. 7
Studies have found that soluble Si polymerizes quickly and that disease development is suppressed only if Si is present in soluble form (Samuels et al., 1991b). To minimize disease development, Si must be provided continuously in the nutrient feed in hydroponic systems.8
Therefore, a continuous source of soluble siliconis very important to combat pathogens. This can be from constant feeding in hydroponics or from retention in the growing medium with soils or soilless mixes.
Optimum ppm of Si in Solution
Liquid Silicon as Si (not SiO2which is 46.743% Si and 53.25% O) is highly beneficial to plants in the range of 25-100 ppm in the nutrient solution. It is not included, at these levels, in any nutrient concentrates. It needs to be added as a separate component by the grower. Because potassium silicate products are highly alkaloid it is recommended to pre-dilute any Si product in eg. 5- 8Ls of water and pH adjust to 5.8- 6.0 before adding to the nutrient tank/reservoir.
We recommend Si use in coco substrate at the lower end of the scale this being between 25 - 35ppm. In inert medias and water-based systems we recommend Si use at higher rates of between 50 75ppm.
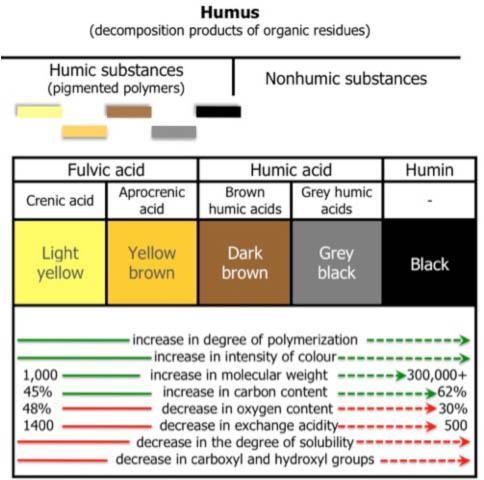
Further, it has been shown that Si uptake is aided by the presence of fulvic acid in solution. 9
References
1."Silicon nutrition in plants" Plant Health Care,Inc.: 1. 12. Retrieved 1 July 2011.
2.W. Voogt and C. Sonneveld (2001) Silicon in horticultural crops grown in soilless culture http://dx.doi.org/10.1016/j.bbr.2011.03.031
3. M.H. Adatia and R.T. Besford (1986) The Effects of Silicon on Cucumber Plants Grown in Recirculating Nutrient Solution
4. R.Shetty, B. Jensen, N. P. Shetty, M. Hansen, C. W. Hansen, K. R. Starkey, H. J. L. Jorgensen (2011) Silicon induced resistance against powdery mildew of roses caused by Podosphaera pannosa
5. Pat Brown, Jim Menzies, and David Ehret (1992) Soluble Silicon Sprays Inhibit Powdery Mildew Development on Grape Leaves
6. Taiichiro Hattori, Shinobu Inanaga, Eiichi Tanimoto, Alexander Lux, Miroslava Luxová and Yukihiro Sugimoto (2003) Silicon-Induced Changes in Viscoelastic Properties of Sorghum Root Cell Walls
7. M. Cherif, N. Benhamous, J. G. Menzies, and R.R. Belanger (1992) Silicon induced resistance in cucumber plants against Pythium ultimum
8. Pat Bowen, Jim Menzies, and David Ehret (1992) Soluble Silicon Sprays Inhibit Powdery Mildew Development on Grape Leaves
9. Chen. C and Wang. X (2007) Sorption of Th (IV) to silica as a function of pH, humic/fulvic acid, ionic strength, electrolyte type.
ADDITIONAL CHELATORS (eg. Amino Acids, Fulvic Acid, Citric Acid etc) IN SOLUTION
In order to understand the benefits of chelation it is necessary to understand some theory around the subject. So, here we go!
Overview
The use of chelates in hydroponic nutrient formulation is critical for optimized nutrient uptake and transport. This is particularly true for the microelements such as Iron.
Micronutrients are crucial partners (cofactors) of enzymes in every metabolic function of the plant. Respiration, photosynthesis, protein synthesis, energy transfer, cell division and cell elongation are all dependent on an adequate supply of calcium, iron, copper, manganese, magnesium, zinc and other micronutrients.
Many microelements in their basic form are unavailable to the plant. This is largely due to the fact that metal microelements such as iron, copper and zinc are positively charged (cations) while the pores (openings) on the plants' leaves and roots are negatively charged. As a result, the positively charged microelements are repelled by the negatively charged plant pores. When a chelate is added with an element like iron it surrounds the microelement and changes the overall charge into a negative or neutral state allowing the element to enter the plant.
Another reason that some microelements require chelating is due to their stability in solution. For instance, iron (Fe) is a reactive metal. In concentrated solution it will react with other fertilizer elements, particularly phosphorus (P) to form an insoluble compound. It forms the compound iron(III) phosphate which is a solid precipitate in water, so it falls out of solution. To prevent this happening Fe used in fertilizers is usually provided in the chelated form.
A chelate describes a kind of organic chemical complex in which a metal ion is bonded/held so tightly that it cannot be changed through contact with other substances, which could convert it to an insoluble form. For instance, Iron (Fe) is a reactive metal. In concentrated solution it will react with other fertiliser elements, particularly phosphorus (P) to form an insoluble compound. Being insoluble iron cannot stay dissolved, so it falls out of solution. To prevent this happening, the iron (Fe) used in fertilizers is provided in chelated form, typically as the synthetic chelates EDTA, DTPA and/or EDDHA. Other than this, iron (Fe) is relatively unavailable to the plant due to the electrical charge that it carries and its reactions with the chemical composition of the root environment. Chelation, thus, makes iron more bioavailable and prevents reactions with other elements.
The typical chelates used in off the shelf hydroponic formulations are copper (Cu), zinc (Zn), Manganese (Mn), Iron (Fe). Nutrients may also contain chelated calcium (Ca), cobalt (Co), nickel (Ni), and magnesium (Mg).
Not all nutrients can be chelated. The positively charged cations, Iron, zinc, copper, manganese, calcium, potassium and magnesium can be chelated while other nutrients (the negatively charged anions) cannot.
CHELATED MINERALS VERSUS COMPLEXED MINERALS
Some nutrient elements only have the ability to be partially surrounded by a chelator/chelating agent and are referred to as a "complexes", while those that are capable of being completely surrounded are termed chelates.
Confusion and often contradictory information exists surrounding chelated and complexed minerals. Terms such as amino acid complexes, amino acid chelates, polysaccharide complexes, lignosulfonate complexes, and amino proteinates abound. In some cases chelates are referred to as complexed or chelate complexes while in other cases fertilizer producers wrongly promote complexed minerals as chelated. This only serves to confuse further. However, the difference between chelated and complexed can be understood via some basic principles.
ALL CHELATES ARE COMPLEXES - NOT ALL COMPLEXES ARE CHELATES
In order for a compound to be called a true chelating agent, it must have certain chemical characteristics. This chelating compound must consist of at least two sites capable of donating electrons (coordinate covalent bond) to the metal it chelates. For true chelation to occur the donating atom(s) must also be in a position within the chelating molecule so that a formation of a ring with the metal ion can occur.
The term complexed originates from combinations of minerals and organic compounds that do not meet the guidelines of a true chelate.
The key difference between a chelated mineral and complexed mineral is that chelates are relatively more stable under adverse conditions while complexes are less thermostable and release the atom quickly under adverse conditions.
Not all nutrients can be chelated. The positively charged cations iron, zinc, copper, cobalt, nickel, manganese, calcium, magnesium, and potassium can be chelated while the negatively charged anions such as phosphorous cannot.
While the negatively charged anions cannot be chelated they can be complexed via the use of donor atoms such as e.g. oxygen, nitrogen or sulphur. Amino acids such as glycine and/or lysergine are often used to complex the anions. Similarly, fulvic acid can be used to complex the negatively charged anions.
Both chelated and complexed minerals are more bioavailable than non-chelated and non-complexed minerals. This makes the use of additional organic (e.g. amino acid, fulvic acid) and inorganic chelators/complexers (eg, EDTA) highly beneficial in hydroponic solutions.
EDTA, DTPA, EDDHA: Synthetic Chelates
Well-formulated hydroponic nutrients ensure that there is a high level of nutrient availability in the correct forms and ratios. Nutrition that offers a diverse range of bioavailable elements will prove more effective than nutrition that has less diversity, particularly where trace elements (metals) are concerned. For this reason combinations of organic and synthetic chelates are demonstrated to benefit yields. What this means in simple terms is that for optimal nutrient bioavailability and uptake both synthetic and organic chelators should be present in solution.
This said
The common types of chelates used by most hydro nutrient manufacturers are the synthetic chelates, EDTA (ethylenediaminetetraacetic acid) and to a lesser extent DTPA (Diethylene triamine pentaacetic acid). Chelates such as EDTA and DTPA have a high affinity for e.g. iron and generally form stable complexes with the metal across a pH range from 4 to 7.
Chelates have several points of attachment with which they "grasp" the trace element. EDTA has four connecting points while DTPA has five. Higher numbers of connection points isnt always an advantage. In some cases the four connection points may hold the element too tightly, while in a different situation these may not hold it tight enough. For this reason, various chelates may prove better than others based on the ion that is chelated and the conditions in which the chelate is present.
For instance, the effectiveness of a chelating agent can depend on pH. For example, EDTA holds iron well up to pH 6, but between pH 6 and pH 8 it progressively loses iron and replaces it with calcium. In the case of iron Fe, EDTA is best suited to slightly lower than neutral pH levels while Fe DTPA is most effective at higher pH values. DTPA is more costly than EDTA and less soluble and is usually found in higher quality fertilizers. DTPA is stable up to a pH of 7.5 while EDTA is stable up to a pH of approximately 6.5.
The most effective of the synthetic chelating agents is ethylenediaminedihydroxy-phenylaceticacid (EDDHA). It is important to note that EDDHA can be formed only with iron and not with other essential microelements such as Cu, Zn, Mn etc. Iron EDDHA is the most stable of all the commonly available iron chelates. This synthetic chelate is held in a bond up to 100 times tighter than DTPA because it has six molecular bonds rather than five bonds. Typically EDDHA is only found in premium fertilizers because of its higher cost. EDDHA is stable up to pH 9.0 (pH range = 4- 9) but is not suitable for foliar applications due to EDDHA only being absorbed through the roots of the plant.
In most cases combinations of chelating agents can improve stability and broaden product effectiveness. That is, a mix/blend of EDTA, DTPA, EDDHA or EDTA and DTPA in formulation best ensures nutrient availability over a wide range of conditions, including those above or below optimal. For this reason, even in hydroponic growing environments where optimum pH (water temperatures etc) can be monitored and maintained there are benefits gained from using a blend of chelated elements in formulation.
Synthetic Chelators and Foliar Feeding
In spraying micro nutrient solutions on the foliage, there might be a danger in using a chelate (such as EDTA) as the chelate may bind to the calcium from the plant tissue (the middle lamella of the cells and the cytoplast membranes contain Ca). Free EDTA might extract the Ca from its sight in the leaf or root membranes and may inflict far more damage than the supply of the metal that is added and is intended to be beneficial. 1
In short, EDTA has a very high affinity for calcium.2As a result, the synthetic chelate will scavenge existing free calcium from the surrounding environment, including cell walls and membranes.
This has the potential to cause the collapse of the cell walls and the leakage of cell contents, leading to phytotoxicity effects.3
For this reason foliar sprays that contain synthetic chelators such as iron EDTA are best avoided. Foliar sprays that contain amino acids or lignosulfonate chelators/complexers, on the other hand, are highly recommended. Well talk more about these chelators/complexers shortly.
- Meeting. Extracted from http://departments.agri.huji.ac.il/fieldcrops/topics_irrigation/uzifert/7thmeet.htm
- Jeppsen, R. (1999) Advantages of Metal Amino Acid Chelates in Foliar Absorption. Proc. Albions International Conference on Plant Nutrition. 16-28.
- Salisbury, F.B, and C.W. Ross. 1992. Plant Pathology Fourth Edition, (Belmont California: Wadsworth Publishing
Chelate Biochemistry Organic vs. Synthetic
The permeability of the synthetic chelates has been shown to differ depending upon the size of their molecules. Larger molecules such as EDTA, DTPA and EDDHA will penetrate the root at a slower rate compared to natural chelating agents (Kannan, 1969). However, cell membranes do not have the capacity to absorb synthetic chelates. For the mineral to be absorbed into the cell, chelates must release the mineral outside of the cell. After the mineral has been released it becomes chelated again by organic acids such as citric acid, malonic acid, tartaric acid, and amino acids (e.g. glycine) that occur naturally within the plant. This secondary chelation process then enables nutrients to move freely inside the plant to areas where they are most needed.
Organic chelators differ to synthetic chelators. An organic chelate, unlike a synthetic chelate, can be uptaken, along with the nutrient element and enter the cell of the plant. This offers distinct advantages to nutrient uptake and translocation.
For this reason, the use of organic chelators can prove beneficial to yields.
ORGANIC CHELATORS
​AMINO ACIDS
Amino Acids Overview
Amino acids are the "building blocks" of protein, without which the formation of any living tissue is impossible.
Amino Acids are fundamental ingredients in the process of Protein Synthesis. About 20 important Amino Acids are involved in the process of each function. Studies have shown that Amino Acids can directly or indirectly influence the physiological activities of the plant.
Amino acids can be taken up directly by the roots. 1Additionally, amino acids have been shown in numerous studies to be beneficial in foliar applications.2, 3, 4
Thus, amino acids used in fertilizer and foliar formulations can increase bioavailability and translocation of essential mineral elements.
Only L-Amino Acids have metabolic activity in plants. D - Amino Acids are not recognised by the enzymatic locus (any of numerous proteins or conjugated proteins produced by living organisms and functioning as biochemical catalysts) and therefore cannot participate in protein synthesis.
L - Glycine & L - Glutamic Acid are known to be very effective chelating agents.
L Glycine is now used to chelate minerals. These chelates are known as glycinates, proteinates, and/or amino proteinates.
GLYCINATES/ PROTEINATES
To touch on glycinate/amino proteinate theory.
Glycine is the simplest amino acid with a molecular weight of 75. Chelates of glycine with cations such as iron, zinc, and copper have been extensively studied.
Glycinates typically contain 2 moles of ligand (glycine) and one mole of metal. The plant recognises this molecule as a protein like nitrogen, allowing it to travel to the growing points such as flowers, fruit and berries where is it required.
Micro nutrients in glycinate/proteinate form have a very stable structure. They can be easily absorbed (uptaken) and directly join the biochemical processes in the plant.
BENEFITS
The requirement of amino acids/glycinates in essential quantities is well known to increase yield and overall quality of crops.
For instance, research conducted in USSR by Tronov and co-workers established that glycinates greatly stimulate the growth of plants. The results concluded that zinc glycinate increased the total, stem, root, and foliage weights by 194, 215, 254 and 147%, respectively. Respective effects of manganese glycinate were 79, 108, 110, and 15%.
Research has demonstrated:
1. Glycinates increase the availability of micronutrients compared to common synthetic chelates (e.g. EDTA, DTPA). 5
2. Crops tend to produce higher yields where glycinates are used.
Today, several hydro nutrient and additive manufacturers use glycinates in formulation. Among them or those that I know of are Advanced Nutrients, Cyco Platinum series and ourselves (Manic Botanix).
Refs
- Wolf-Nicolas Fischer, Bruno André, Doris Rentsch, Sylvia Krolkiewicz, Mechthild Tegeder, Kevin Breitkreuz and Wolf B. Frommer (199
Amino acid transport in plants
- A. Ilhami KÖKSAL, Hatice DUMANOGLU, Nurdan Tuna GÜNES, Mehmet AKTAS (199
The Effects of Different Amino Acid Chelate Foliar Fertilizers on Yield, Fruit Quality, Shoot Growth and Fe, Zn, Cu, Mn Content of Leaves in Williams Pear Cultivar (Pyrus communis L.)
- Ashmead, H. (1986) World Nutritional Crisis in Agriculture, Foliar Feeding of Plants with Amino Acid Chelates.
- Jeppsen, R. (1999) Advantages of Metal Amino Acid Chelates in Foliar Absorption. Proc. Albions International Conference on Plant Nutrition. 16-28.
- Y.L Pan and D.W Wang EFFECTS OF IRON ON POTATO GROWTH. Research agronomists, Beijing Academy of Agriculture and Forest Science